Yanwu Xu
South China University of Technology
FCMBench: A Comprehensive Financial Credit Multimodal Benchmark for Real-world Applications
Jan 06, 2026Abstract:As multimodal AI becomes widely used for credit risk assessment and document review, a domain-specific benchmark is urgently needed that (1) reflects documents and workflows specific to financial credit applications, (2) includes credit-specific understanding and real-world robustness, and (3) preserves privacy compliance without sacrificing practical utility. Here, we introduce FCMBench-V1.0 -- a large-scale financial credit multimodal benchmark for real-world applications, covering 18 core certificate types, with 4,043 privacy-compliant images and 8,446 QA samples. The FCMBench evaluation framework consists of three dimensions: Perception, Reasoning, and Robustness, including 3 foundational perception tasks, 4 credit-specific reasoning tasks that require decision-oriented understanding of visual evidence, and 10 real-world acquisition artifact types for robustness stress testing. To reconcile compliance with realism, we construct all samples via a closed synthesis-capture pipeline: we manually synthesize document templates with virtual content and capture scenario-aware images in-house. This design also mitigates pre-training data leakage by avoiding web-sourced or publicly released images. FCMBench can effectively discriminate performance disparities and robustness across modern vision-language models. Extensive experiments were conducted on 23 state-of-the-art vision-language models (VLMs) from 14 top AI companies and research institutes. Among them, Gemini 3 Pro achieves the best F1(\%) score as a commercial model (64.61), Qwen3-VL-235B achieves the best score as an open-source baseline (57.27), and our financial credit-specific model, Qfin-VL-Instruct, achieves the top overall score (64.92). Robustness evaluations show that even top-performing models suffer noticeable performance drops under acquisition artifacts.
Rethinking Diffusion-Based Image Generators for Fundus Fluorescein Angiography Synthesis on Limited Data
Dec 17, 2024Abstract:Fundus imaging is a critical tool in ophthalmology, with different imaging modalities offering unique advantages. For instance, fundus fluorescein angiography (FFA) can accurately identify eye diseases. However, traditional invasive FFA involves the injection of sodium fluorescein, which can cause discomfort and risks. Generating corresponding FFA images from non-invasive fundus images holds significant practical value but also presents challenges. First, limited datasets constrain the performance and effectiveness of models. Second, previous studies have primarily focused on generating FFA for single diseases or single modalities, often resulting in poor performance for patients with various ophthalmic conditions. To address these issues, we propose a novel latent diffusion model-based framework, Diffusion, which introduces a fine-tuning protocol to overcome the challenge of limited medical data and unleash the generative capabilities of diffusion models. Furthermore, we designed a new approach to tackle the challenges of generating across different modalities and disease types. On limited datasets, our framework achieves state-of-the-art results compared to existing methods, offering significant potential to enhance ophthalmic diagnostics and patient care. Our code will be released soon to support further research in this field.
Precision-Enhanced Human-Object Contact Detection via Depth-Aware Perspective Interaction and Object Texture Restoration
Dec 13, 2024



Abstract:Human-object contact (HOT) is designed to accurately identify the areas where humans and objects come into contact. Current methods frequently fail to account for scenarios where objects are frequently blocking the view, resulting in inaccurate identification of contact areas. To tackle this problem, we suggest using a perspective interaction HOT detector called PIHOT, which utilizes a depth map generation model to offer depth information of humans and objects related to the camera, thereby preventing false interaction detection. Furthermore, we use mask dilatation and object restoration techniques to restore the texture details in covered areas, improve the boundaries between objects, and enhance the perception of humans interacting with objects. Moreover, a spatial awareness perception is intended to concentrate on the characteristic features close to the points of contact. The experimental results show that the PIHOT algorithm achieves state-of-the-art performance on three benchmark datasets for HOT detection tasks. Compared to the most recent DHOT, our method enjoys an average improvement of 13%, 27.5%, 16%, and 18.5% on SC-Acc., C-Acc., mIoU, and wIoU metrics, respectively.
SnapGen-V: Generating a Five-Second Video within Five Seconds on a Mobile Device
Dec 13, 2024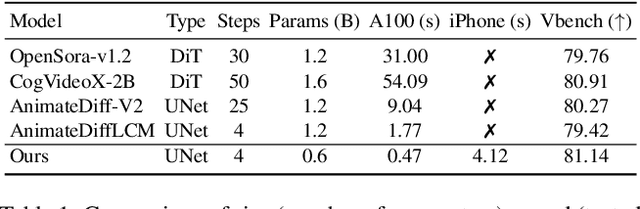
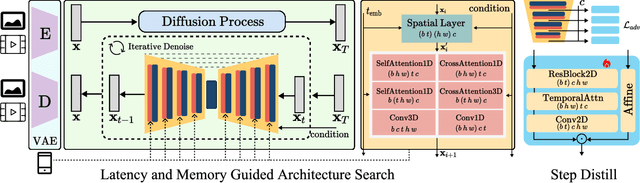
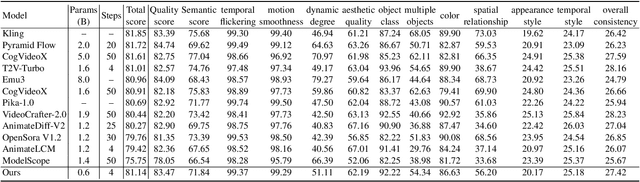
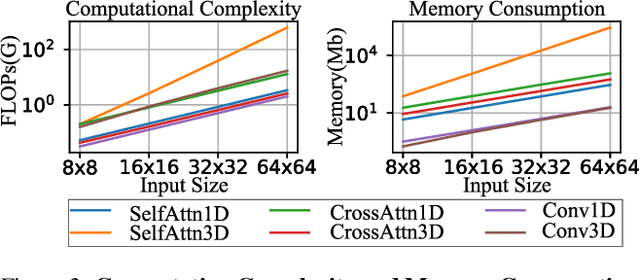
Abstract:We have witnessed the unprecedented success of diffusion-based video generation over the past year. Recently proposed models from the community have wielded the power to generate cinematic and high-resolution videos with smooth motions from arbitrary input prompts. However, as a supertask of image generation, video generation models require more computation and are thus hosted mostly on cloud servers, limiting broader adoption among content creators. In this work, we propose a comprehensive acceleration framework to bring the power of the large-scale video diffusion model to the hands of edge users. From the network architecture scope, we initialize from a compact image backbone and search out the design and arrangement of temporal layers to maximize hardware efficiency. In addition, we propose a dedicated adversarial fine-tuning algorithm for our efficient model and reduce the denoising steps to 4. Our model, with only 0.6B parameters, can generate a 5-second video on an iPhone 16 PM within 5 seconds. Compared to server-side models that take minutes on powerful GPUs to generate a single video, we accelerate the generation by magnitudes while delivering on-par quality.
Semi-IIN: Semi-supervised Intra-inter modal Interaction Learning Network for Multimodal Sentiment Analysis
Dec 13, 2024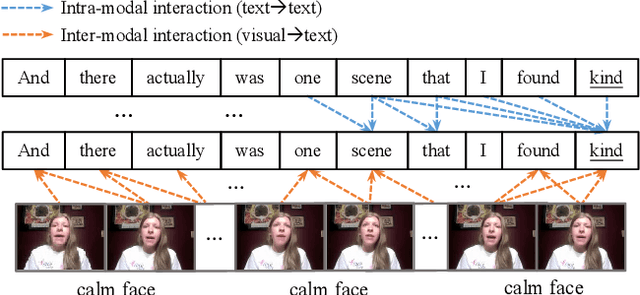
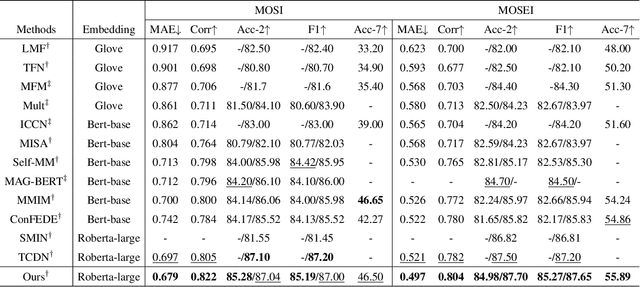
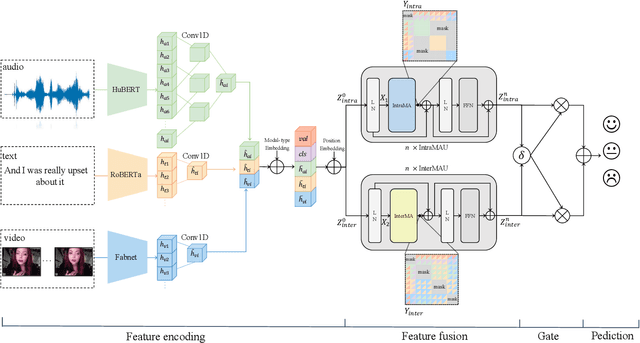
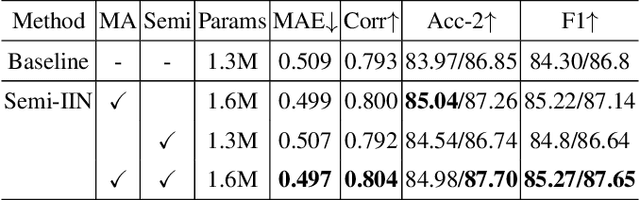
Abstract:Despite multimodal sentiment analysis being a fertile research ground that merits further investigation, current approaches take up high annotation cost and suffer from label ambiguity, non-amicable to high-quality labeled data acquisition. Furthermore, choosing the right interactions is essential because the significance of intra- or inter-modal interactions can differ among various samples. To this end, we propose Semi-IIN, a Semi-supervised Intra-inter modal Interaction learning Network for multimodal sentiment analysis. Semi-IIN integrates masked attention and gating mechanisms, enabling effective dynamic selection after independently capturing intra- and inter-modal interactive information. Combined with the self-training approach, Semi-IIN fully utilizes the knowledge learned from unlabeled data. Experimental results on two public datasets, MOSI and MOSEI, demonstrate the effectiveness of Semi-IIN, establishing a new state-of-the-art on several metrics. Code is available at https://github.com/flow-ljh/Semi-IIN.
SnapGen: Taming High-Resolution Text-to-Image Models for Mobile Devices with Efficient Architectures and Training
Dec 12, 2024Abstract:Existing text-to-image (T2I) diffusion models face several limitations, including large model sizes, slow runtime, and low-quality generation on mobile devices. This paper aims to address all of these challenges by developing an extremely small and fast T2I model that generates high-resolution and high-quality images on mobile platforms. We propose several techniques to achieve this goal. First, we systematically examine the design choices of the network architecture to reduce model parameters and latency, while ensuring high-quality generation. Second, to further improve generation quality, we employ cross-architecture knowledge distillation from a much larger model, using a multi-level approach to guide the training of our model from scratch. Third, we enable a few-step generation by integrating adversarial guidance with knowledge distillation. For the first time, our model SnapGen, demonstrates the generation of 1024x1024 px images on a mobile device around 1.4 seconds. On ImageNet-1K, our model, with only 372M parameters, achieves an FID of 2.06 for 256x256 px generation. On T2I benchmarks (i.e., GenEval and DPG-Bench), our model with merely 379M parameters, surpasses large-scale models with billions of parameters at a significantly smaller size (e.g., 7x smaller than SDXL, 14x smaller than IF-XL).
TED-VITON: Transformer-Empowered Diffusion Models for Virtual Try-On
Nov 26, 2024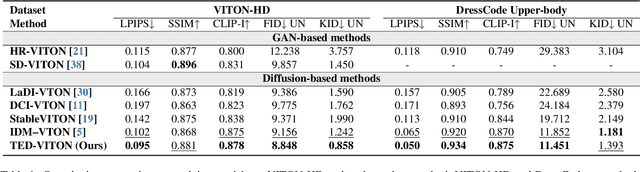
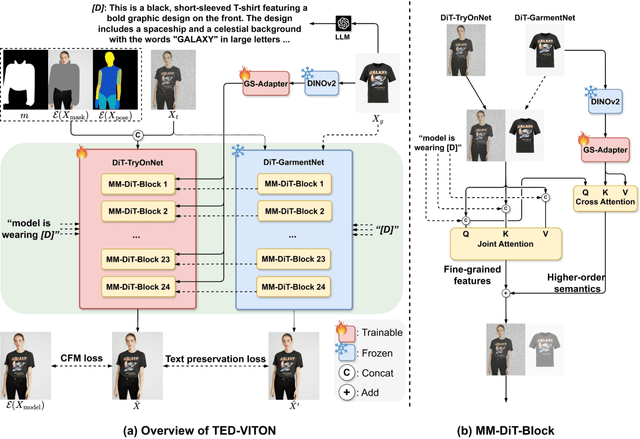


Abstract:Recent advancements in Virtual Try-On (VTO) have demonstrated exceptional efficacy in generating realistic images and preserving garment details, largely attributed to the robust generative capabilities of text-to-image (T2I) diffusion backbones. However, the T2I models that underpin these methods have become outdated, thereby limiting the potential for further improvement in VTO. Additionally, current methods face notable challenges in accurately rendering text on garments without distortion and preserving fine-grained details, such as textures and material fidelity. The emergence of Diffusion Transformer (DiT) based T2I models has showcased impressive performance and offers a promising opportunity for advancing VTO. Directly applying existing VTO techniques to transformer-based T2I models is ineffective due to substantial architectural differences, which hinder their ability to fully leverage the models' advanced capabilities for improved text generation. To address these challenges and unlock the full potential of DiT-based T2I models for VTO, we propose TED-VITON, a novel framework that integrates a Garment Semantic (GS) Adapter for enhancing garment-specific features, a Text Preservation Loss to ensure accurate and distortion-free text rendering, and a constraint mechanism to generate prompts by optimizing Large Language Model (LLM). These innovations enable state-of-the-art (SOTA) performance in visual quality and text fidelity, establishing a new benchmark for VTO task.
Pre-trained Molecular Language Models with Random Functional Group Masking
Nov 03, 2024



Abstract:Recent advancements in computational chemistry have leveraged the power of trans-former-based language models, such as MoLFormer, pre-trained using a vast amount of simplified molecular-input line-entry system (SMILES) sequences, to understand and predict molecular properties and activities, a critical step in fields like drug discovery and materials science. To further improve performance, researchers have introduced graph neural networks with graph-based molecular representations, such as GEM, incorporating the topology, geometry, 2D or even 3D structures of molecules into pre-training. While most of molecular graphs in existing studies were automatically converted from SMILES sequences, it is to assume that transformer-based language models might be able to implicitly learn structure-aware representations from SMILES sequences. In this paper, we propose \ours{} -- a SMILES-based \underline{\em M}olecular \underline{\em L}anguage \underline{\em M}odel, which randomly masking SMILES subsequences corresponding to specific molecular \underline{\em F}unctional \underline{\em G}roups to incorporate structure information of atoms during the pre-training phase. This technique aims to compel the model to better infer molecular structures and properties, thus enhancing its predictive capabilities. Extensive experimental evaluations across 11 benchmark classification and regression tasks in the chemical domain demonstrate the robustness and superiority of \ours{}. Our findings reveal that \ours{} outperforms existing pre-training models, either based on SMILES or graphs, in 9 out of the 11 downstream tasks, ranking as a close second in the remaining ones.
Non-Invasive to Invasive: Enhancing FFA Synthesis from CFP with a Benchmark Dataset and a Novel Network
Oct 19, 2024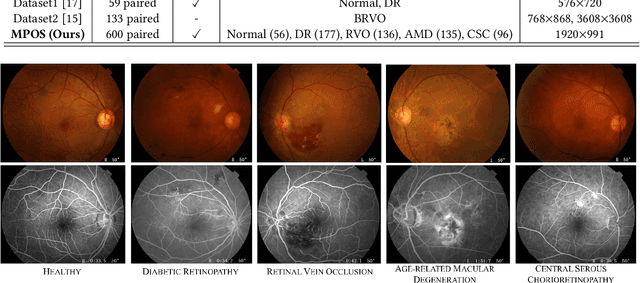
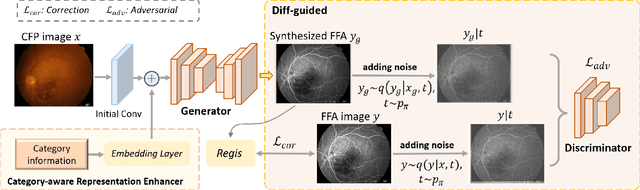
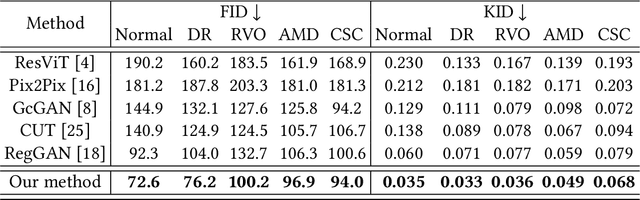
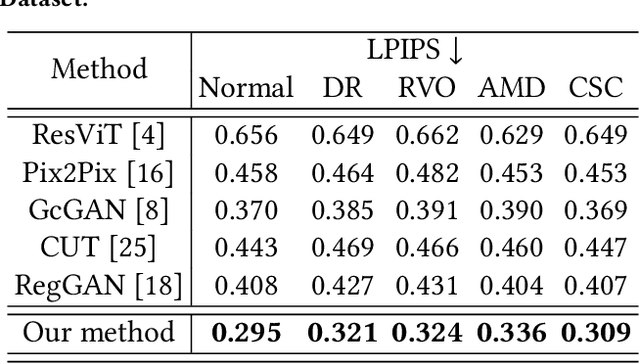
Abstract:Fundus imaging is a pivotal tool in ophthalmology, and different imaging modalities are characterized by their specific advantages. For example, Fundus Fluorescein Angiography (FFA) uniquely provides detailed insights into retinal vascular dynamics and pathology, surpassing Color Fundus Photographs (CFP) in detecting microvascular abnormalities and perfusion status. However, the conventional invasive FFA involves discomfort and risks due to fluorescein dye injection, and it is meaningful but challenging to synthesize FFA images from non-invasive CFP. Previous studies primarily focused on FFA synthesis in a single disease category. In this work, we explore FFA synthesis in multiple diseases by devising a Diffusion-guided generative adversarial network, which introduces an adaptive and dynamic diffusion forward process into the discriminator and adds a category-aware representation enhancer. Moreover, to facilitate this research, we collect the first multi-disease CFP and FFA paired dataset, named the Multi-disease Paired Ocular Synthesis (MPOS) dataset, with four different fundus diseases. Experimental results show that our FFA synthesis network can generate better FFA images compared to state-of-the-art methods. Furthermore, we introduce a paired-modal diagnostic network to validate the effectiveness of synthetic FFA images in the diagnosis of multiple fundus diseases, and the results show that our synthesized FFA images with the real CFP images have higher diagnosis accuracy than that of the compared FFA synthesizing methods. Our research bridges the gap between non-invasive imaging and FFA, thereby offering promising prospects to enhance ophthalmic diagnosis and patient care, with a focus on reducing harm to patients through non-invasive procedures. Our dataset and code will be released to support further research in this field (https://github.com/whq-xxh/FFA-Synthesis).
ETSCL: An Evidence Theory-Based Supervised Contrastive Learning Framework for Multi-modal Glaucoma Grading
Jul 19, 2024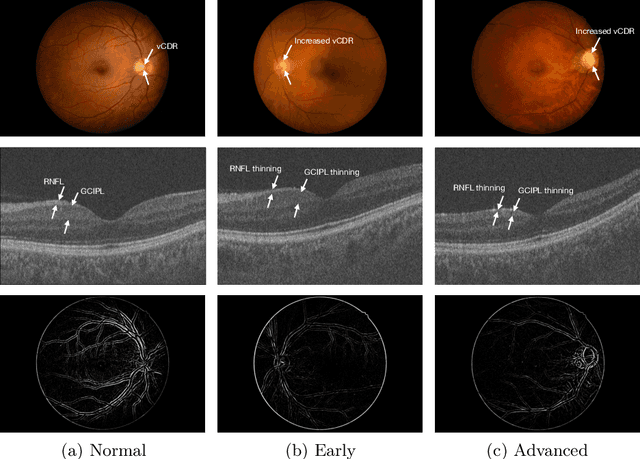
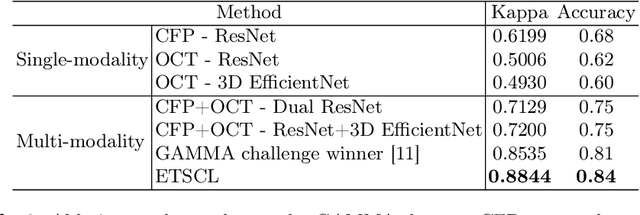
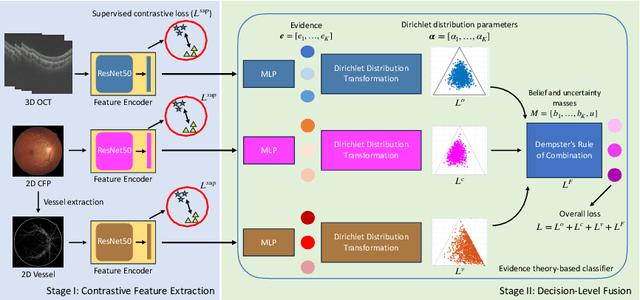
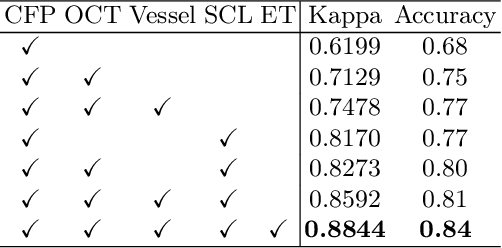
Abstract:Glaucoma is one of the leading causes of vision impairment. Digital imaging techniques, such as color fundus photography (CFP) and optical coherence tomography (OCT), provide quantitative and noninvasive methods for glaucoma diagnosis. Recently, in the field of computer-aided glaucoma diagnosis, multi-modality methods that integrate the CFP and OCT modalities have achieved greater diagnostic accuracy compared to single-modality methods. However, it remains challenging to extract reliable features due to the high similarity of medical images and the unbalanced multi-modal data distribution. Moreover, existing methods overlook the uncertainty estimation of different modalities, leading to unreliable predictions. To address these challenges, we propose a novel framework, namely ETSCL, which consists of a contrastive feature extraction stage and a decision-level fusion stage. Specifically, the supervised contrastive loss is employed to enhance the discriminative power in the feature extraction process, resulting in more effective features. In addition, we utilize the Frangi vesselness algorithm as a preprocessing step to incorporate vessel information to assist in the prediction. In the decision-level fusion stage, an evidence theory-based multi-modality classifier is employed to combine multi-source information with uncertainty estimation. Extensive experiments demonstrate that our method achieves state-of-the-art performance. The code is available at \url{https://github.com/master-Shix/ETSCL}.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge