Qingqing Tang
Non-Invasive to Invasive: Enhancing FFA Synthesis from CFP with a Benchmark Dataset and a Novel Network
Oct 19, 2024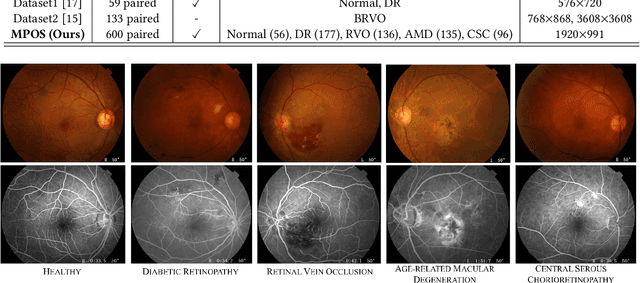
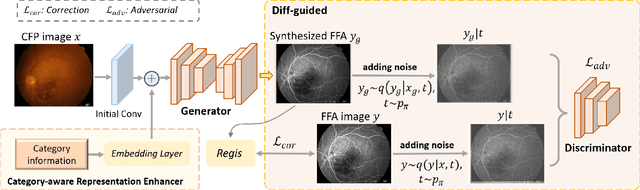
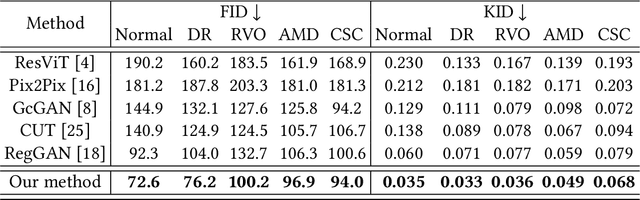
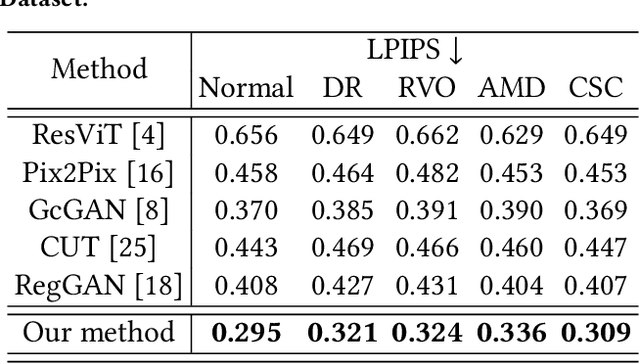
Abstract:Fundus imaging is a pivotal tool in ophthalmology, and different imaging modalities are characterized by their specific advantages. For example, Fundus Fluorescein Angiography (FFA) uniquely provides detailed insights into retinal vascular dynamics and pathology, surpassing Color Fundus Photographs (CFP) in detecting microvascular abnormalities and perfusion status. However, the conventional invasive FFA involves discomfort and risks due to fluorescein dye injection, and it is meaningful but challenging to synthesize FFA images from non-invasive CFP. Previous studies primarily focused on FFA synthesis in a single disease category. In this work, we explore FFA synthesis in multiple diseases by devising a Diffusion-guided generative adversarial network, which introduces an adaptive and dynamic diffusion forward process into the discriminator and adds a category-aware representation enhancer. Moreover, to facilitate this research, we collect the first multi-disease CFP and FFA paired dataset, named the Multi-disease Paired Ocular Synthesis (MPOS) dataset, with four different fundus diseases. Experimental results show that our FFA synthesis network can generate better FFA images compared to state-of-the-art methods. Furthermore, we introduce a paired-modal diagnostic network to validate the effectiveness of synthetic FFA images in the diagnosis of multiple fundus diseases, and the results show that our synthesized FFA images with the real CFP images have higher diagnosis accuracy than that of the compared FFA synthesizing methods. Our research bridges the gap between non-invasive imaging and FFA, thereby offering promising prospects to enhance ophthalmic diagnosis and patient care, with a focus on reducing harm to patients through non-invasive procedures. Our dataset and code will be released to support further research in this field (https://github.com/whq-xxh/FFA-Synthesis).
Advancing UWF-SLO Vessel Segmentation with Source-Free Active Domain Adaptation and a Novel Multi-Center Dataset
Jun 19, 2024Abstract:Accurate vessel segmentation in Ultra-Wide-Field Scanning Laser Ophthalmoscopy (UWF-SLO) images is crucial for diagnosing retinal diseases. Although recent techniques have shown encouraging outcomes in vessel segmentation, models trained on one medical dataset often underperform on others due to domain shifts. Meanwhile, manually labeling high-resolution UWF-SLO images is an extremely challenging, time-consuming and expensive task. In response, this study introduces a pioneering framework that leverages a patch-based active domain adaptation approach. By actively recommending a few valuable image patches by the devised Cascade Uncertainty-Predominance (CUP) selection strategy for labeling and model-finetuning, our method significantly improves the accuracy of UWF-SLO vessel segmentation across diverse medical centers. In addition, we annotate and construct the first Multi-center UWF-SLO Vessel Segmentation (MU-VS) dataset to promote this topic research, comprising data from multiple institutions. This dataset serves as a valuable resource for cross-center evaluation, verifying the effectiveness and robustness of our approach. Experimental results demonstrate that our approach surpasses existing domain adaptation and active learning methods, considerably reducing the gap between the Upper and Lower bounds with minimal annotations, highlighting our method's practical clinical value. We will release our dataset and code to facilitate relevant research: https://github.com/whq-xxh/SFADA-UWF-SLO.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge