Jerry L. Prince
Likelihood-Separable Diffusion Inference for Multi-Image MRI Super-Resolution
Jan 20, 2026Abstract:Diffusion models are the current state-of-the-art for solving inverse problems in imaging. Their impressive generative capability allows them to approximate sampling from a prior distribution, which alongside a known likelihood function permits posterior sampling without retraining the model. While recent methods have made strides in advancing the accuracy of posterior sampling, the majority focuses on single-image inverse problems. However, for modalities such as magnetic resonance imaging (MRI), it is common to acquire multiple complementary measurements, each low-resolution along a different axis. In this work, we generalize common diffusion-based inverse single-image problem solvers for multi-image super-resolution (MISR) MRI. We show that the DPS likelihood correction allows an exactly-separable gradient decomposition across independently acquired measurements, enabling MISR without constructing a joint operator, modifying the diffusion model, or increasing network function evaluations. We derive MISR versions of DPS, DMAP, DPPS, and diffusion-based PnP/ADMM, and demonstrate substantial gains over SISR across $4\times/8\times/16\times$ anisotropic degradations. Our results achieve state-of-the-art super-resolution of anisotropic MRI volumes and, critically, enable reconstruction of near-isotropic anatomy from routine 2D multi-slice acquisitions, which are otherwise highly degraded in orthogonal views.
MSRepaint: Multiple Sclerosis Repaint with Conditional Denoising Diffusion Implicit Model for Bidirectional Lesion Filling and Synthesis
Oct 02, 2025Abstract:In multiple sclerosis, lesions interfere with automated magnetic resonance imaging analyses such as brain parcellation and deformable registration, while lesion segmentation models are hindered by the limited availability of annotated training data. To address both issues, we propose MSRepaint, a unified diffusion-based generative model for bidirectional lesion filling and synthesis that restores anatomical continuity for downstream analyses and augments segmentation through realistic data generation. MSRepaint conditions on spatial lesion masks for voxel-level control, incorporates contrast dropout to handle missing inputs, integrates a repainting mechanism to preserve surrounding anatomy during lesion filling and synthesis, and employs a multi-view DDIM inversion and fusion pipeline for 3D consistency with fast inference. Extensive evaluations demonstrate the effectiveness of MSRepaint across multiple tasks. For lesion filling, we evaluate both the accuracy within the filled regions and the impact on downstream tasks including brain parcellation and deformable registration. MSRepaint outperforms the traditional lesion filling methods FSL and NiftySeg, and achieves accuracy on par with FastSurfer-LIT, a recent diffusion model-based inpainting method, while offering over 20 times faster inference. For lesion synthesis, state-of-the-art MS lesion segmentation models trained on MSRepaint-synthesized data outperform those trained on CarveMix-synthesized data or real ISBI challenge training data across multiple benchmarks, including the MICCAI 2016 and UMCL datasets. Additionally, we demonstrate that MSRepaint's unified bidirectional filling and synthesis capability, with full spatial control over lesion appearance, enables high-fidelity simulation of lesion evolution in longitudinal MS progression.
Segmenting Thalamic Nuclei: T1 Maps Provide a Reliable and Efficient Solution
Aug 17, 2025Abstract:Accurate thalamic nuclei segmentation is crucial for understanding neurological diseases, brain functions, and guiding clinical interventions. However, the optimal inputs for segmentation remain unclear. This study systematically evaluates multiple MRI contrasts, including MPRAGE and FGATIR sequences, quantitative PD and T1 maps, and multiple T1-weighted images at different inversion times (multi-TI), to determine the most effective inputs. For multi-TI images, we employ a gradient-based saliency analysis with Monte Carlo dropout and propose an Overall Importance Score to select the images contributing most to segmentation. A 3D U-Net is trained on each of these configurations. Results show that T1 maps alone achieve strong quantitative performance and superior qualitative outcomes, while PD maps offer no added value. These findings underscore the value of T1 maps as a reliable and efficient input among the evaluated options, providing valuable guidance for optimizing imaging protocols when thalamic structures are of clinical or research interest.
UNISELF: A Unified Network with Instance Normalization and Self-Ensembled Lesion Fusion for Multiple Sclerosis Lesion Segmentation
Aug 06, 2025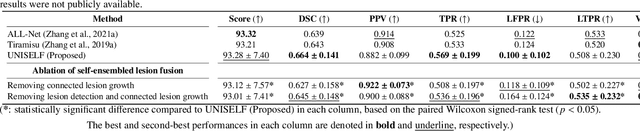
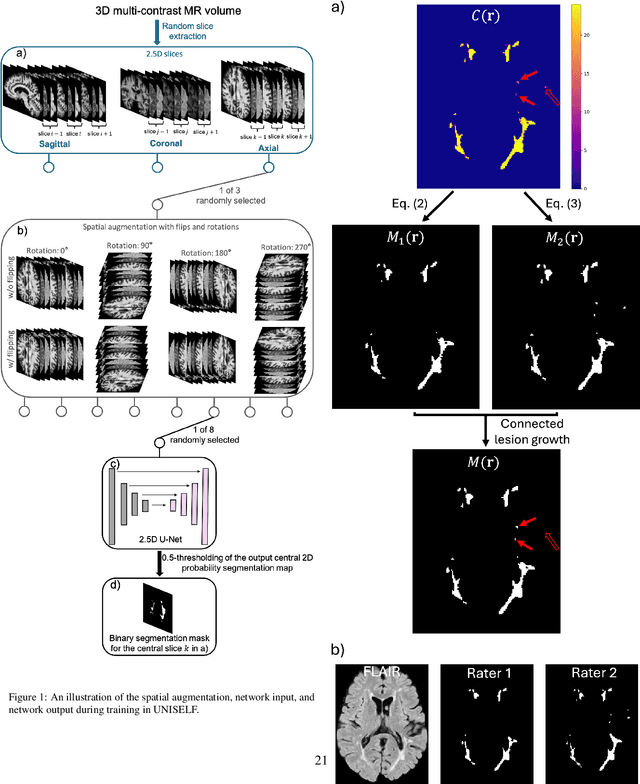
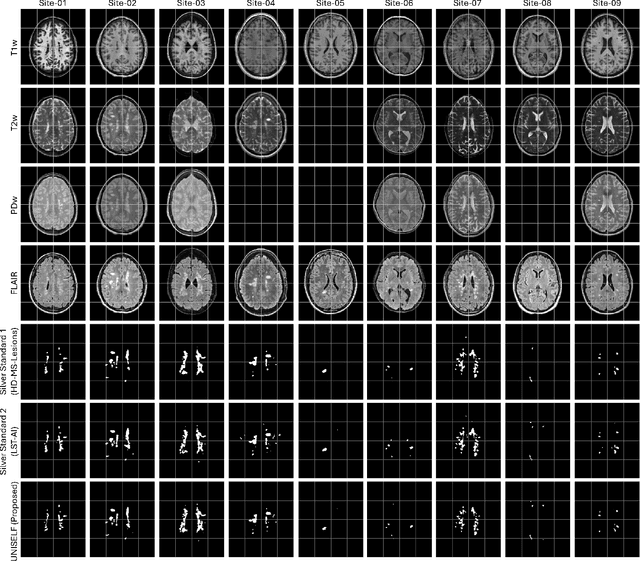

Abstract:Automated segmentation of multiple sclerosis (MS) lesions using multicontrast magnetic resonance (MR) images improves efficiency and reproducibility compared to manual delineation, with deep learning (DL) methods achieving state-of-the-art performance. However, these DL-based methods have yet to simultaneously optimize in-domain accuracy and out-of-domain generalization when trained on a single source with limited data, or their performance has been unsatisfactory. To fill this gap, we propose a method called UNISELF, which achieves high accuracy within a single training domain while demonstrating strong generalizability across multiple out-of-domain test datasets. UNISELF employs a novel test-time self-ensembled lesion fusion to improve segmentation accuracy, and leverages test-time instance normalization (TTIN) of latent features to address domain shifts and missing input contrasts. Trained on the ISBI 2015 longitudinal MS segmentation challenge training dataset, UNISELF ranks among the best-performing methods on the challenge test dataset. Additionally, UNISELF outperforms all benchmark methods trained on the same ISBI training data across diverse out-of-domain test datasets with domain shifts and missing contrasts, including the public MICCAI 2016 and UMCL datasets, as well as a private multisite dataset. These test datasets exhibit domain shifts and/or missing contrasts caused by variations in acquisition protocols, scanner types, and imaging artifacts arising from imperfect acquisition. Our code is available at https://github.com/uponacceptance.
Beyond the LUMIR challenge: The pathway to foundational registration models
May 30, 2025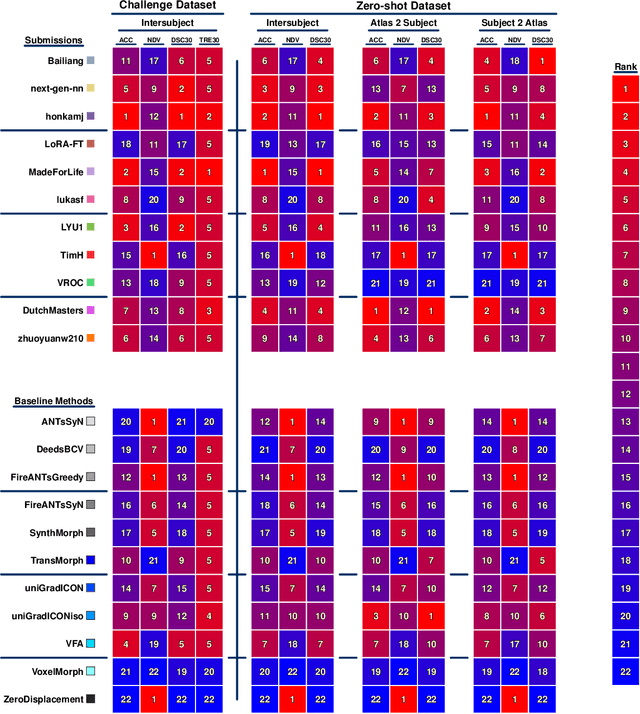
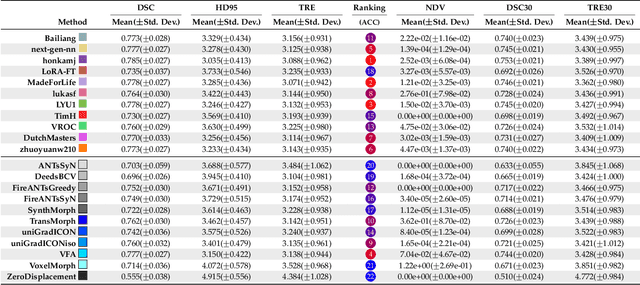
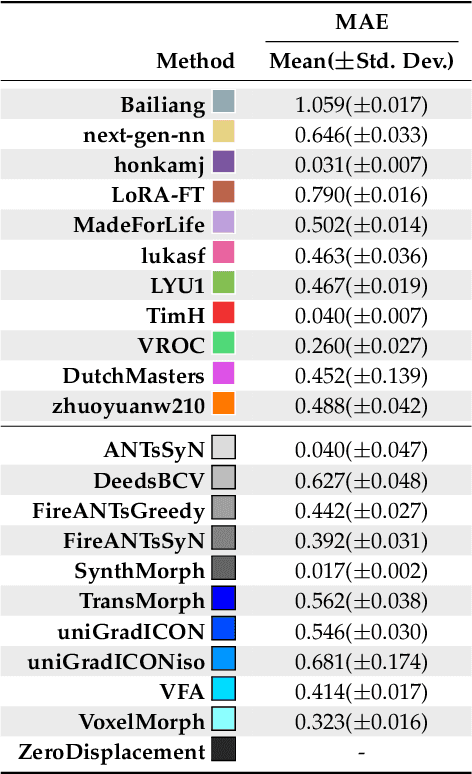
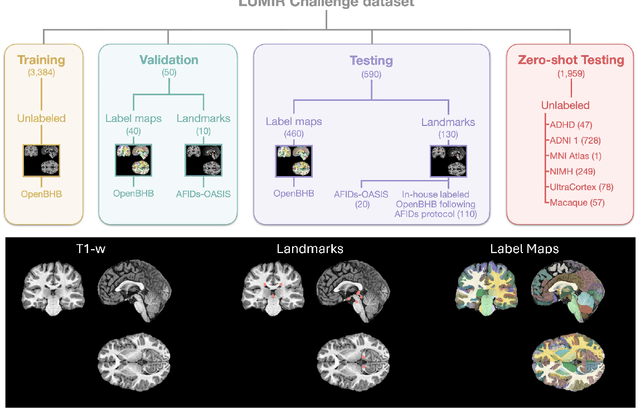
Abstract:Medical image challenges have played a transformative role in advancing the field, catalyzing algorithmic innovation and establishing new performance standards across diverse clinical applications. Image registration, a foundational task in neuroimaging pipelines, has similarly benefited from the Learn2Reg initiative. Building on this foundation, we introduce the Large-scale Unsupervised Brain MRI Image Registration (LUMIR) challenge, a next-generation benchmark designed to assess and advance unsupervised brain MRI registration. Distinct from prior challenges that leveraged anatomical label maps for supervision, LUMIR removes this dependency by providing over 4,000 preprocessed T1-weighted brain MRIs for training without any label maps, encouraging biologically plausible deformation modeling through self-supervision. In addition to evaluating performance on 590 held-out test subjects, LUMIR introduces a rigorous suite of zero-shot generalization tasks, spanning out-of-domain imaging modalities (e.g., FLAIR, T2-weighted, T2*-weighted), disease populations (e.g., Alzheimer's disease), acquisition protocols (e.g., 9.4T MRI), and species (e.g., macaque brains). A total of 1,158 subjects and over 4,000 image pairs were included for evaluation. Performance was assessed using both segmentation-based metrics (Dice coefficient, 95th percentile Hausdorff distance) and landmark-based registration accuracy (target registration error). Across both in-domain and zero-shot tasks, deep learning-based methods consistently achieved state-of-the-art accuracy while producing anatomically plausible deformation fields. The top-performing deep learning-based models demonstrated diffeomorphic properties and inverse consistency, outperforming several leading optimization-based methods, and showing strong robustness to most domain shifts, the exception being a drop in performance on out-of-domain contrasts.
Brightness-Invariant Tracking Estimation in Tagged MRI
May 23, 2025Abstract:Magnetic resonance (MR) tagging is an imaging technique for noninvasively tracking tissue motion in vivo by creating a visible pattern of magnetization saturation (tags) that deforms with the tissue. Due to longitudinal relaxation and progression to steady-state, the tags and tissue brightnesses change over time, which makes tracking with optical flow methods error-prone. Although Fourier methods can alleviate these problems, they are also sensitive to brightness changes as well as spectral spreading due to motion. To address these problems, we introduce the brightness-invariant tracking estimation (BRITE) technique for tagged MRI. BRITE disentangles the anatomy from the tag pattern in the observed tagged image sequence and simultaneously estimates the Lagrangian motion. The inherent ill-posedness of this problem is addressed by leveraging the expressive power of denoising diffusion probabilistic models to represent the probabilistic distribution of the underlying anatomy and the flexibility of physics-informed neural networks to estimate biologically-plausible motion. A set of tagged MR images of a gel phantom was acquired with various tag periods and imaging flip angles to demonstrate the impact of brightness variations and to validate our method. The results show that BRITE achieves more accurate motion and strain estimates as compared to other state of the art methods, while also being resistant to tag fading.
A Speech-to-Video Synthesis Approach Using Spatio-Temporal Diffusion for Vocal Tract MRI
Mar 15, 2025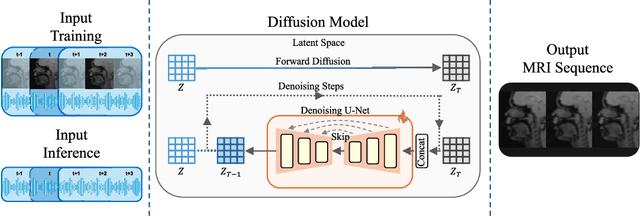
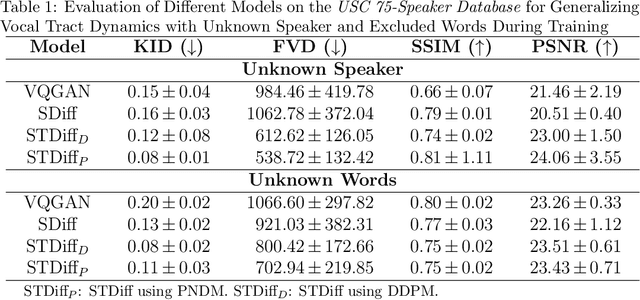
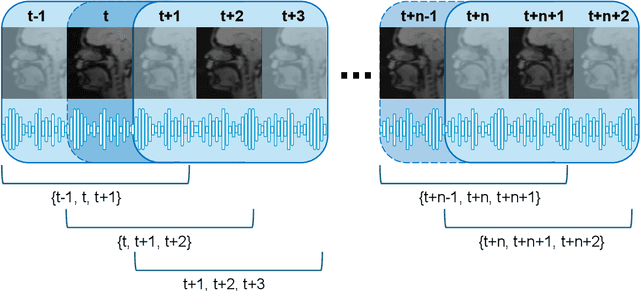

Abstract:Understanding the relationship between vocal tract motion during speech and the resulting acoustic signal is crucial for aided clinical assessment and developing personalized treatment and rehabilitation strategies. Toward this goal, we introduce an audio-to-video generation framework for creating Real Time/cine-Magnetic Resonance Imaging (RT-/cine-MRI) visuals of the vocal tract from speech signals. Our framework first preprocesses RT-/cine-MRI sequences and speech samples to achieve temporal alignment, ensuring synchronization between visual and audio data. We then employ a modified stable diffusion model, integrating structural and temporal blocks, to effectively capture movement characteristics and temporal dynamics in the synchronized data. This process enables the generation of MRI sequences from new speech inputs, improving the conversion of audio into visual data. We evaluated our framework on healthy controls and tongue cancer patients by analyzing and comparing the vocal tract movements in synthesized videos. Our framework demonstrated adaptability to new speech inputs and effective generalization. In addition, positive human evaluations confirmed its effectiveness, with realistic and accurate visualizations, suggesting its potential for outpatient therapy and personalized simulation of vocal tract visualizations.
ECLARE: Efficient cross-planar learning for anisotropic resolution enhancement
Mar 14, 2025Abstract:In clinical imaging, magnetic resonance (MR) image volumes are often acquired as stacks of 2D slices, permitting decreased scan times, improved signal-to-noise ratio, and image contrasts unique to 2D MR pulse sequences. While this is sufficient for clinical evaluation, automated algorithms designed for 3D analysis perform sub-optimally on 2D-acquired scans, especially those with thick slices and gaps between slices. Super-resolution (SR) methods aim to address this problem, but previous methods do not address all of the following: slice profile shape estimation, slice gap, domain shift, and non-integer / arbitrary upsampling factors. In this paper, we propose ECLARE (Efficient Cross-planar Learning for Anisotropic Resolution Enhancement), a self-SR method that addresses each of these factors. ECLARE estimates the slice profile from the 2D-acquired multi-slice MR volume, trains a network to learn the mapping from low-resolution to high-resolution in-plane patches from the same volume, and performs SR with anti-aliasing. We compared ECLARE to cubic B-spline interpolation, SMORE, and other contemporary SR methods. We used realistic and representative simulations so that quantitative performance against a ground truth could be computed, and ECLARE outperformed all other methods in both signal recovery and downstream tasks. On real data for which there is no ground truth, ECLARE demonstrated qualitative superiority over other methods as well. Importantly, as ECLARE does not use external training data it cannot suffer from domain shift between training and testing. Our code is open-source and available at https://www.github.com/sremedios/eclare.
Pitfalls of defacing whole-head MRI: re-identification risk with diffusion models and compromised research potential
Jan 31, 2025Abstract:Defacing is often applied to head magnetic resonance image (MRI) datasets prior to public release to address privacy concerns. The alteration of facial and nearby voxels has provoked discussions about the true capability of these techniques to ensure privacy as well as their impact on downstream tasks. With advancements in deep generative models, the extent to which defacing can protect privacy is uncertain. Additionally, while the altered voxels are known to contain valuable anatomical information, their potential to support research beyond the anatomical regions directly affected by defacing remains uncertain. To evaluate these considerations, we develop a refacing pipeline that recovers faces in defaced head MRIs using cascaded diffusion probabilistic models (DPMs). The DPMs are trained on images from 180 subjects and tested on images from 484 unseen subjects, 469 of whom are from a different dataset. To assess whether the altered voxels in defacing contain universally useful information, we also predict computed tomography (CT)-derived skeletal muscle radiodensity from facial voxels in both defaced and original MRIs. The results show that DPMs can generate high-fidelity faces that resemble the original faces from defaced images, with surface distances to the original faces significantly smaller than those of a population average face (p < 0.05). This performance also generalizes well to previously unseen datasets. For skeletal muscle radiodensity predictions, using defaced images results in significantly weaker Spearman's rank correlation coefficients compared to using original images (p < 10-4). For shin muscle, the correlation is statistically significant (p < 0.05) when using original images but not statistically significant (p > 0.05) when any defacing method is applied, suggesting that defacing might not only fail to protect privacy but also eliminate valuable information.
Unique MS Lesion Identification from MRI
Oct 12, 2024



Abstract:Unique identification of multiple sclerosis (MS) white matter lesions (WMLs) is important to help characterize MS progression. WMLs are routinely identified from magnetic resonance images (MRIs) but the resultant total lesion load does not correlate well with EDSS; whereas mean unique lesion volume has been shown to correlate with EDSS. Our approach builds on prior work by incorporating Hessian matrix computation from lesion probability maps before using the random walker algorithm to estimate the volume of each unique lesion. Synthetic images demonstrate our ability to accurately count the number of lesions present. The takeaways, are: 1) that our method correctly identifies all lesions including many that are missed by previous methods; 2) we can better separate confluent lesions; and 3) we can accurately capture the total volume of WMLs in a given probability map. This work will allow new more meaningful statistics to be computed from WMLs in brain MRIs
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge