Blake E. Dewey
Likelihood-Separable Diffusion Inference for Multi-Image MRI Super-Resolution
Jan 20, 2026Abstract:Diffusion models are the current state-of-the-art for solving inverse problems in imaging. Their impressive generative capability allows them to approximate sampling from a prior distribution, which alongside a known likelihood function permits posterior sampling without retraining the model. While recent methods have made strides in advancing the accuracy of posterior sampling, the majority focuses on single-image inverse problems. However, for modalities such as magnetic resonance imaging (MRI), it is common to acquire multiple complementary measurements, each low-resolution along a different axis. In this work, we generalize common diffusion-based inverse single-image problem solvers for multi-image super-resolution (MISR) MRI. We show that the DPS likelihood correction allows an exactly-separable gradient decomposition across independently acquired measurements, enabling MISR without constructing a joint operator, modifying the diffusion model, or increasing network function evaluations. We derive MISR versions of DPS, DMAP, DPPS, and diffusion-based PnP/ADMM, and demonstrate substantial gains over SISR across $4\times/8\times/16\times$ anisotropic degradations. Our results achieve state-of-the-art super-resolution of anisotropic MRI volumes and, critically, enable reconstruction of near-isotropic anatomy from routine 2D multi-slice acquisitions, which are otherwise highly degraded in orthogonal views.
Segmenting Thalamic Nuclei: T1 Maps Provide a Reliable and Efficient Solution
Aug 17, 2025Abstract:Accurate thalamic nuclei segmentation is crucial for understanding neurological diseases, brain functions, and guiding clinical interventions. However, the optimal inputs for segmentation remain unclear. This study systematically evaluates multiple MRI contrasts, including MPRAGE and FGATIR sequences, quantitative PD and T1 maps, and multiple T1-weighted images at different inversion times (multi-TI), to determine the most effective inputs. For multi-TI images, we employ a gradient-based saliency analysis with Monte Carlo dropout and propose an Overall Importance Score to select the images contributing most to segmentation. A 3D U-Net is trained on each of these configurations. Results show that T1 maps alone achieve strong quantitative performance and superior qualitative outcomes, while PD maps offer no added value. These findings underscore the value of T1 maps as a reliable and efficient input among the evaluated options, providing valuable guidance for optimizing imaging protocols when thalamic structures are of clinical or research interest.
UNISELF: A Unified Network with Instance Normalization and Self-Ensembled Lesion Fusion for Multiple Sclerosis Lesion Segmentation
Aug 06, 2025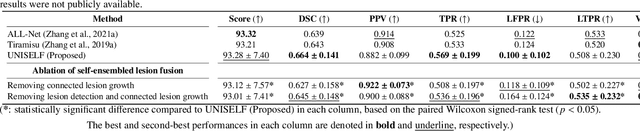
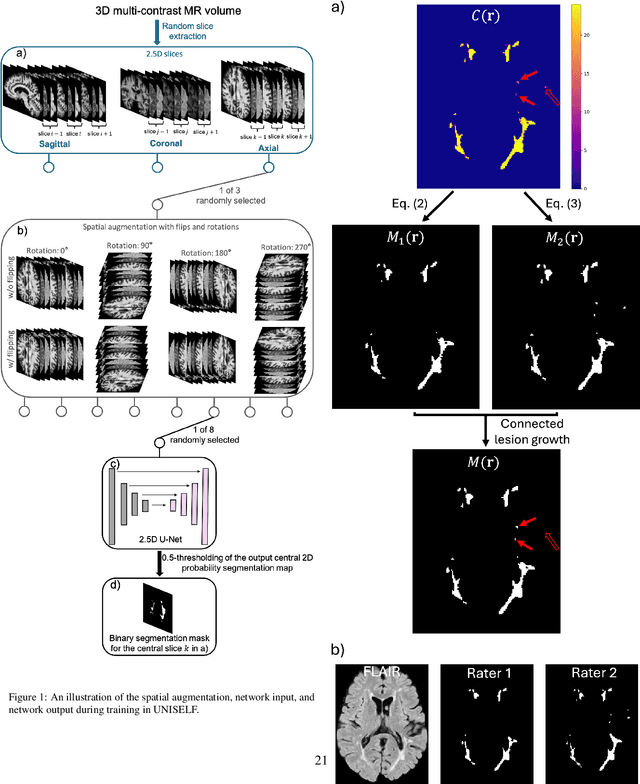
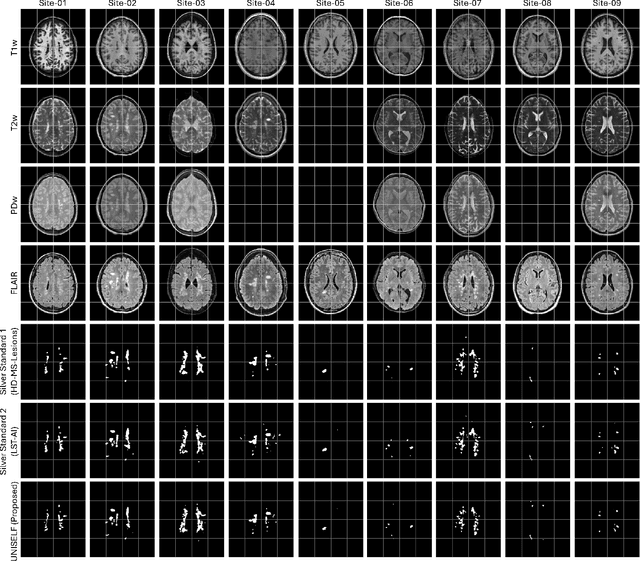

Abstract:Automated segmentation of multiple sclerosis (MS) lesions using multicontrast magnetic resonance (MR) images improves efficiency and reproducibility compared to manual delineation, with deep learning (DL) methods achieving state-of-the-art performance. However, these DL-based methods have yet to simultaneously optimize in-domain accuracy and out-of-domain generalization when trained on a single source with limited data, or their performance has been unsatisfactory. To fill this gap, we propose a method called UNISELF, which achieves high accuracy within a single training domain while demonstrating strong generalizability across multiple out-of-domain test datasets. UNISELF employs a novel test-time self-ensembled lesion fusion to improve segmentation accuracy, and leverages test-time instance normalization (TTIN) of latent features to address domain shifts and missing input contrasts. Trained on the ISBI 2015 longitudinal MS segmentation challenge training dataset, UNISELF ranks among the best-performing methods on the challenge test dataset. Additionally, UNISELF outperforms all benchmark methods trained on the same ISBI training data across diverse out-of-domain test datasets with domain shifts and missing contrasts, including the public MICCAI 2016 and UMCL datasets, as well as a private multisite dataset. These test datasets exhibit domain shifts and/or missing contrasts caused by variations in acquisition protocols, scanner types, and imaging artifacts arising from imperfect acquisition. Our code is available at https://github.com/uponacceptance.
Synthetic multi-inversion time magnetic resonance images for visualization of subcortical structures
Jun 04, 2025Abstract:Purpose: Visualization of subcortical gray matter is essential in neuroscience and clinical practice, particularly for disease understanding and surgical planning.While multi-inversion time (multi-TI) T$_1$-weighted (T$_1$-w) magnetic resonance (MR) imaging improves visualization, it is rarely acquired in clinical settings. Approach: We present SyMTIC (Synthetic Multi-TI Contrasts), a deep learning method that generates synthetic multi-TI images using routinely acquired T$_1$-w, T$_2$-weighted (T$_2$-w), and FLAIR images. Our approach combines image translation via deep neural networks with imaging physics to estimate longitudinal relaxation time (T$_1$) and proton density (PD) maps. These maps are then used to compute multi-TI images with arbitrary inversion times. Results: SyMTIC was trained using paired MPRAGE and FGATIR images along with T$_2$-w and FLAIR images. It accurately synthesized multi-TI images from standard clinical inputs, achieving image quality comparable to that from explicitly acquired multi-TI data.The synthetic images, especially for TI values between 400-800 ms, enhanced visualization of subcortical structures and improved segmentation of thalamic nuclei. Conclusion: SyMTIC enables robust generation of high-quality multi-TI images from routine MR contrasts. It generalizes well to varied clinical datasets, including those with missing FLAIR images or unknown parameters, offering a practical solution for improving brain MR image visualization and analysis.
ECLARE: Efficient cross-planar learning for anisotropic resolution enhancement
Mar 14, 2025Abstract:In clinical imaging, magnetic resonance (MR) image volumes are often acquired as stacks of 2D slices, permitting decreased scan times, improved signal-to-noise ratio, and image contrasts unique to 2D MR pulse sequences. While this is sufficient for clinical evaluation, automated algorithms designed for 3D analysis perform sub-optimally on 2D-acquired scans, especially those with thick slices and gaps between slices. Super-resolution (SR) methods aim to address this problem, but previous methods do not address all of the following: slice profile shape estimation, slice gap, domain shift, and non-integer / arbitrary upsampling factors. In this paper, we propose ECLARE (Efficient Cross-planar Learning for Anisotropic Resolution Enhancement), a self-SR method that addresses each of these factors. ECLARE estimates the slice profile from the 2D-acquired multi-slice MR volume, trains a network to learn the mapping from low-resolution to high-resolution in-plane patches from the same volume, and performs SR with anti-aliasing. We compared ECLARE to cubic B-spline interpolation, SMORE, and other contemporary SR methods. We used realistic and representative simulations so that quantitative performance against a ground truth could be computed, and ECLARE outperformed all other methods in both signal recovery and downstream tasks. On real data for which there is no ground truth, ECLARE demonstrated qualitative superiority over other methods as well. Importantly, as ECLARE does not use external training data it cannot suffer from domain shift between training and testing. Our code is open-source and available at https://www.github.com/sremedios/eclare.
RATNUS: Rapid, Automatic Thalamic Nuclei Segmentation using Multimodal MRI inputs
Sep 10, 2024



Abstract:Accurate segmentation of thalamic nuclei is important for better understanding brain function and improving disease treatment. Traditional segmentation methods often rely on a single T1-weighted image, which has limited contrast in the thalamus. In this work, we introduce RATNUS, which uses synthetic T1-weighted images with many inversion times along with diffusion-derived features to enhance the visibility of nuclei within the thalamus. Using these features, a convolutional neural network is used to segment 13 thalamic nuclei. For comparison with other methods, we introduce a unified nuclei labeling scheme. Our results demonstrate an 87.19% average true positive rate (TPR) against manual labeling. In comparison, FreeSurfer and THOMAS achieve TPRs of 64.25% and 57.64%, respectively, demonstrating the superiority of RATNUS in thalamic nuclei segmentation.
Beyond MR Image Harmonization: Resolution Matters Too
Aug 30, 2024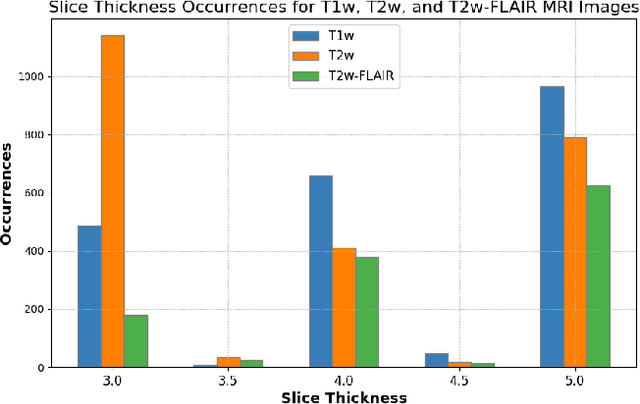
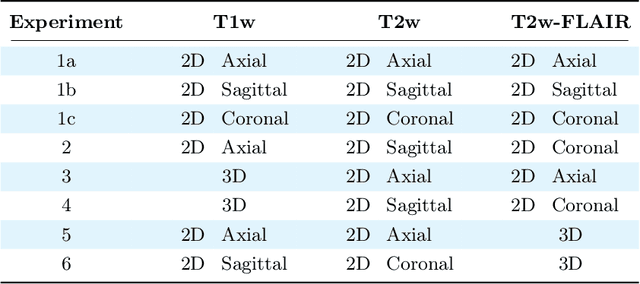
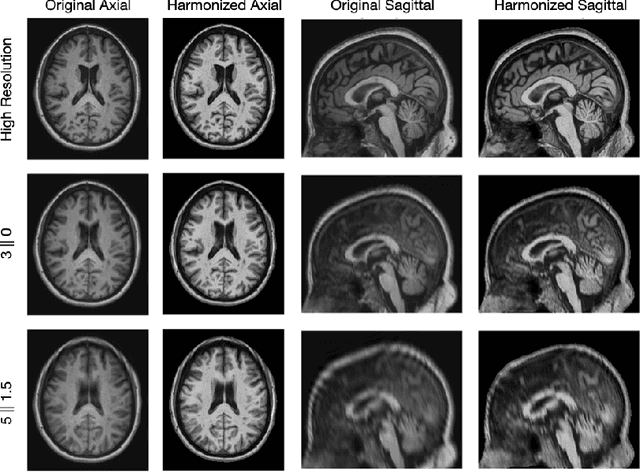
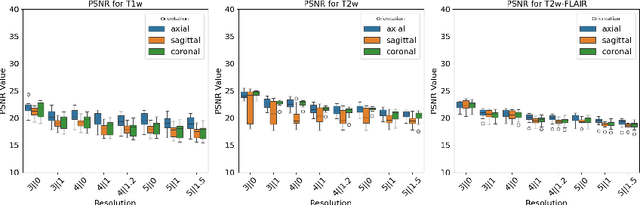
Abstract:Magnetic resonance (MR) imaging is commonly used in the clinical setting to non-invasively monitor the body. There exists a large variability in MR imaging due to differences in scanner hardware, software, and protocol design. Ideally, a processing algorithm should perform robustly to this variability, but that is not always the case in reality. This introduces a need for image harmonization to overcome issues of domain shift when performing downstream analysis such as segmentation. Most image harmonization models focus on acquisition parameters such as inversion time or repetition time, but they ignore an important aspect in MR imaging -- resolution. In this paper, we evaluate the impact of image resolution on harmonization using a pretrained harmonization algorithm. We simulate 2D acquisitions of various slice thicknesses and gaps from 3D acquired, 1mm3 isotropic MR images and demonstrate how the performance of a state-of-the-art image harmonization algorithm varies as resolution changes. We discuss the most ideal scenarios for image resolution including acquisition orientation when 3D imaging is not available, which is common for many clinical scanners. Our results show that harmonization on low-resolution images does not account for acquisition resolution and orientation variations. Super-resolution can be used to alleviate resolution variations but it is not always used. Our methodology can generalize to help evaluate the impact of image acquisition resolution for multiple tasks. Determining the limits of a pretrained algorithm is important when considering preprocessing steps and trust in the results.
Super-Resolution Multi-Contrast Unbiased Eye Atlases With Deep Probabilistic Refinement
Jan 05, 2024



Abstract:Eye morphology varies significantly across the population, especially for the orbit and optic nerve. These variations limit the feasibility and robustness of generalizing population-wise features of eye organs to an unbiased spatial reference. To tackle these limitations, we propose a process for creating high-resolution unbiased eye atlases. First, to restore spatial details from scans with a low through-plane resolution compared to a high in-plane resolution, we apply a deep learning-based super-resolution algorithm. Then, we generate an initial unbiased reference with an iterative metric-based registration using a small portion of subject scans. We register the remaining scans to this template and refine the template using an unsupervised deep probabilistic approach that generates a more expansive deformation field to enhance the organ boundary alignment. We demonstrate this framework using magnetic resonance images across four different MRI tissue contrasts, generating four atlases in separate spatial alignments. For each tissue contrast, we find a significant improvement in the average Dice score across four labeled regions compared to a standard registration framework consisting of rigid, affine, and deformable transformations. These results highlight the effective alignment of eye organs and boundaries using our proposed process. By combining super-resolution preprocessing and deep probabilistic models, we address the challenge of generating an eye atlas to serve as a standardized reference across a largely variable population.
AniRes2D: Anisotropic Residual-enhanced Diffusion for 2D MR Super-Resolution
Dec 07, 2023Abstract:Anisotropic low-resolution (LR) magnetic resonance (MR) images are fast to obtain but hinder automated processing. We propose to use denoising diffusion probabilistic models (DDPMs) to super-resolve these 2D-acquired LR MR slices. This paper introduces AniRes2D, a novel approach combining DDPM with a residual prediction for 2D super-resolution (SR). Results demonstrate that AniRes2D outperforms several other DDPM-based models in quantitative metrics, visual quality, and out-of-domain evaluation. We use a trained AniRes2D to super-resolve 3D volumes slice by slice, where comparative quantitative results and reduced skull aliasing are achieved compared to a recent state-of-the-art self-supervised 3D super-resolution method. Furthermore, we explored the use of noise conditioning augmentation (NCA) as an alternative augmentation technique for DDPM-based SR models, but it was found to reduce performance. Our findings contribute valuable insights to the application of DDPMs for SR of anisotropic MR images.
Towards an accurate and generalizable multiple sclerosis lesion segmentation model using self-ensembled lesion fusion
Dec 03, 2023



Abstract:Automatic multiple sclerosis (MS) lesion segmentation using multi-contrast magnetic resonance (MR) images provides improved efficiency and reproducibility compared to manual delineation. Current state-of-the-art automatic MS lesion segmentation methods utilize modified U-Net-like architectures. However, in the literature, dedicated architecture modifications were always required to maximize their performance. In addition, the best-performing methods have not proven to be generalizable to diverse test datasets with contrast variations and image artifacts. In this work, we developed an accurate and generalizable MS lesion segmentation model using the well-known U-Net architecture without further modification. A novel test-time self-ensembled lesion fusion strategy is proposed that not only achieved the best performance using the ISBI 2015 MS segmentation challenge data but also demonstrated robustness across various self-ensemble parameter choices. Moreover, equipped with instance normalization rather than batch normalization widely used in literature, the model trained on the ISBI challenge data generalized well on clinical test datasets from different scanners.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge