Yutong Xie
MedViz: An Agent-based, Visual-guided Research Assistant for Navigating Biomedical Literature
Jan 28, 2026Abstract:Biomedical researchers face increasing challenges in navigating millions of publications in diverse domains. Traditional search engines typically return articles as ranked text lists, offering little support for global exploration or in-depth analysis. Although recent advances in generative AI and large language models have shown promise in tasks such as summarization, extraction, and question answering, their dialog-based implementations are poorly integrated with literature search workflows. To address this gap, we introduce MedViz, a visual analytics system that integrates multiple AI agents with interactive visualization to support the exploration of the large-scale biomedical literature. MedViz combines a semantic map of millions of articles with agent-driven functions for querying, summarizing, and hypothesis generation, allowing researchers to iteratively refine questions, identify trends, and uncover hidden connections. By bridging intelligent agents with interactive visualization, MedViz transforms biomedical literature search into a dynamic, exploratory process that accelerates knowledge discovery.
Robust Atypical Mitosis Classification with DenseNet121: Stain-Aware Augmentation and Hybrid Loss for Domain Generalization
Oct 26, 2025Abstract:Atypical mitotic figures are important biomarkers of tumor aggressiveness in histopathology, yet reliable recognition remains challenging due to severe class imbalance and variability across imaging domains. We present a DenseNet-121-based framework tailored for atypical mitosis classification in the MIDOG 2025 (Track 2) setting. Our method integrates stain-aware augmentation (Macenko), geometric and intensity transformations, and imbalance-aware learning via weighted sampling with a hybrid objective combining class-weighted binary cross-entropy and focal loss. Trained end-to-end with AdamW and evaluated across multiple independent domains, the model demonstrates strong generalization under scanner and staining shifts, achieving balanced accuracy 85.0%, AUROC 0.927, sensitivity 89.2%, and specificity 80.9% on the official test set. These results indicate that combining DenseNet-121 with stain-aware augmentation and imbalance-adaptive objectives yields a robust, domain-generalizable framework for atypical mitosis classification suitable for real-world computational pathology workflows.
A Knowledge-driven Adaptive Collaboration of LLMs for Enhancing Medical Decision-making
Sep 18, 2025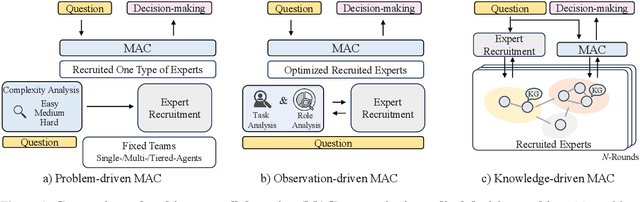
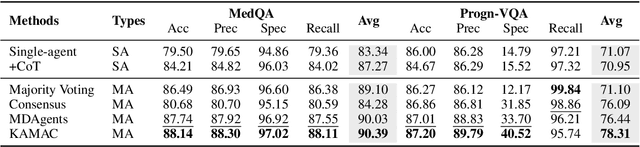
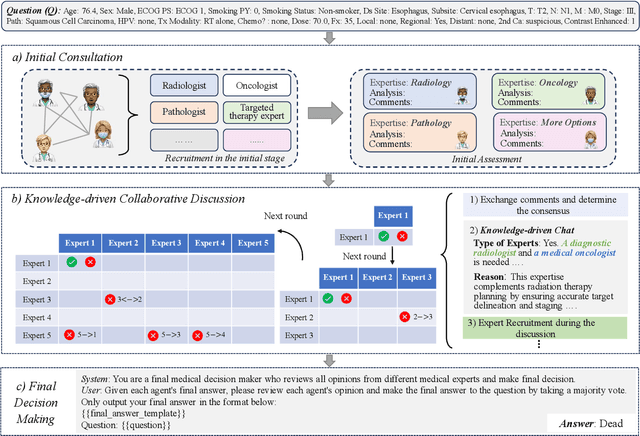
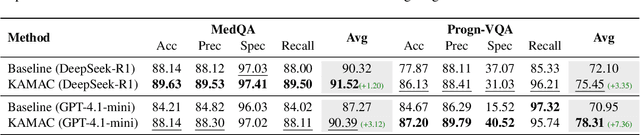
Abstract:Medical decision-making often involves integrating knowledge from multiple clinical specialties, typically achieved through multidisciplinary teams. Inspired by this collaborative process, recent work has leveraged large language models (LLMs) in multi-agent collaboration frameworks to emulate expert teamwork. While these approaches improve reasoning through agent interaction, they are limited by static, pre-assigned roles, which hinder adaptability and dynamic knowledge integration. To address these limitations, we propose KAMAC, a Knowledge-driven Adaptive Multi-Agent Collaboration framework that enables LLM agents to dynamically form and expand expert teams based on the evolving diagnostic context. KAMAC begins with one or more expert agents and then conducts a knowledge-driven discussion to identify and fill knowledge gaps by recruiting additional specialists as needed. This supports flexible, scalable collaboration in complex clinical scenarios, with decisions finalized through reviewing updated agent comments. Experiments on two real-world medical benchmarks demonstrate that KAMAC significantly outperforms both single-agent and advanced multi-agent methods, particularly in complex clinical scenarios (i.e., cancer prognosis) requiring dynamic, cross-specialty expertise. Our code is publicly available at: https://github.com/XiaoXiao-Woo/KAMAC.
How Effectively Can Large Language Models Connect SNP Variants and ECG Phenotypes for Cardiovascular Risk Prediction?
Aug 10, 2025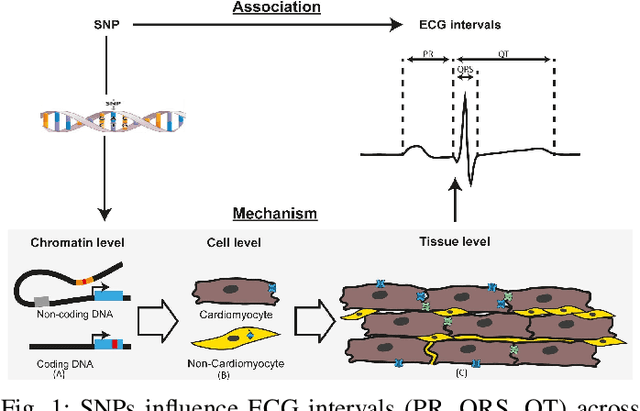
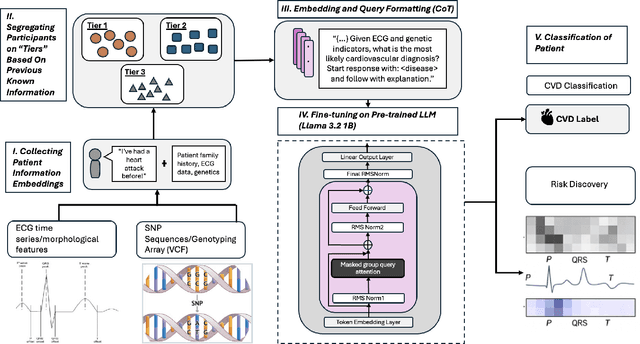
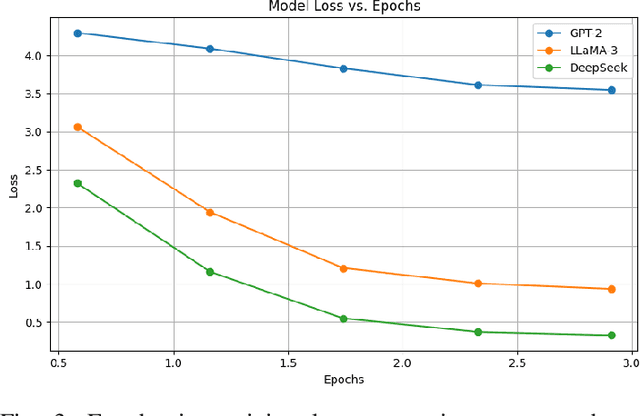
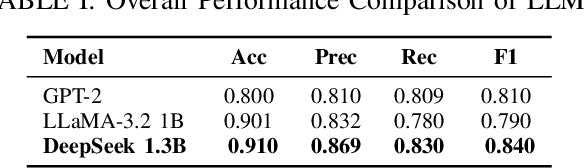
Abstract:Cardiovascular disease (CVD) prediction remains a tremendous challenge due to its multifactorial etiology and global burden of morbidity and mortality. Despite the growing availability of genomic and electrophysiological data, extracting biologically meaningful insights from such high-dimensional, noisy, and sparsely annotated datasets remains a non-trivial task. Recently, LLMs has been applied effectively to predict structural variations in biological sequences. In this work, we explore the potential of fine-tuned LLMs to predict cardiac diseases and SNPs potentially leading to CVD risk using genetic markers derived from high-throughput genomic profiling. We investigate the effect of genetic patterns associated with cardiac conditions and evaluate how LLMs can learn latent biological relationships from structured and semi-structured genomic data obtained by mapping genetic aspects that are inherited from the family tree. By framing the problem as a Chain of Thought (CoT) reasoning task, the models are prompted to generate disease labels and articulate informed clinical deductions across diverse patient profiles and phenotypes. The findings highlight the promise of LLMs in contributing to early detection, risk assessment, and ultimately, the advancement of personalized medicine in cardiac care.
TransPrune: Token Transition Pruning for Efficient Large Vision-Language Model
Jul 28, 2025Abstract:Large Vision-Language Models (LVLMs) have advanced multimodal learning but face high computational costs due to the large number of visual tokens, motivating token pruning to improve inference efficiency. The key challenge lies in identifying which tokens are truly important. Most existing approaches rely on attention-based criteria to estimate token importance. However, they inherently suffer from certain limitations, such as positional bias. In this work, we explore a new perspective on token importance based on token transitions in LVLMs. We observe that the transition of token representations provides a meaningful signal of semantic information. Based on this insight, we propose TransPrune, a training-free and efficient token pruning method. Specifically, TransPrune progressively prunes tokens by assessing their importance through a combination of Token Transition Variation (TTV)-which measures changes in both the magnitude and direction of token representations-and Instruction-Guided Attention (IGA), which measures how strongly the instruction attends to image tokens via attention. Extensive experiments demonstrate that TransPrune achieves comparable multimodal performance to original LVLMs, such as LLaVA-v1.5 and LLaVA-Next, across eight benchmarks, while reducing inference TFLOPs by more than half. Moreover, TTV alone can serve as an effective criterion without relying on attention, achieving performance comparable to attention-based methods. The code will be made publicly available upon acceptance of the paper at https://github.com/liaolea/TransPrune.
SAM-aware Test-time Adaptation for Universal Medical Image Segmentation
Jun 05, 2025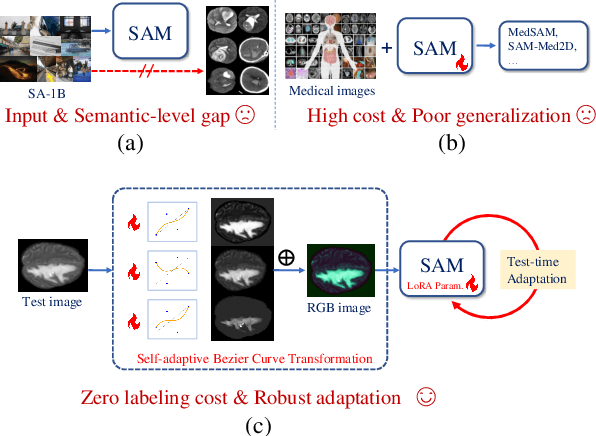
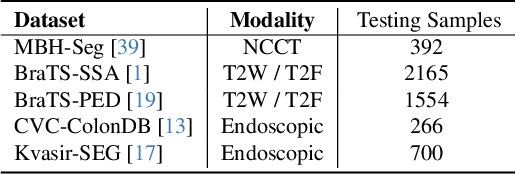
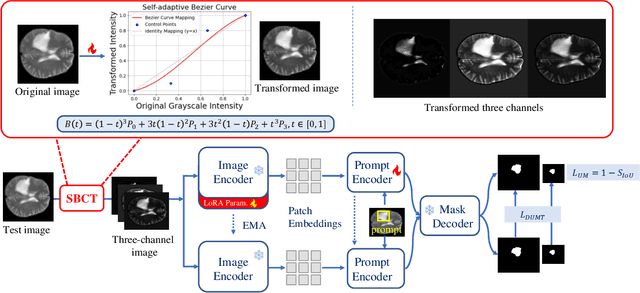
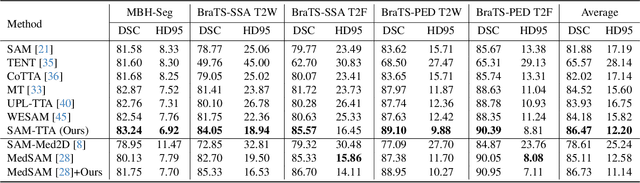
Abstract:Universal medical image segmentation using the Segment Anything Model (SAM) remains challenging due to its limited adaptability to medical domains. Existing adaptations, such as MedSAM, enhance SAM's performance in medical imaging but at the cost of reduced generalization to unseen data. Therefore, in this paper, we propose SAM-aware Test-Time Adaptation (SAM-TTA), a fundamentally different pipeline that preserves the generalization of SAM while improving its segmentation performance in medical imaging via a test-time framework. SAM-TTA tackles two key challenges: (1) input-level discrepancies caused by differences in image acquisition between natural and medical images and (2) semantic-level discrepancies due to fundamental differences in object definition between natural and medical domains (e.g., clear boundaries vs. ambiguous structures). Specifically, our SAM-TTA framework comprises (1) Self-adaptive Bezier Curve-based Transformation (SBCT), which adaptively converts single-channel medical images into three-channel SAM-compatible inputs while maintaining structural integrity, to mitigate the input gap between medical and natural images, and (2) Dual-scale Uncertainty-driven Mean Teacher adaptation (DUMT), which employs consistency learning to align SAM's internal representations to medical semantics, enabling efficient adaptation without auxiliary supervision or expensive retraining. Extensive experiments on five public datasets demonstrate that our SAM-TTA outperforms existing TTA approaches and even surpasses fully fine-tuned models such as MedSAM in certain scenarios, establishing a new paradigm for universal medical image segmentation. Code can be found at https://github.com/JianghaoWu/SAM-TTA.
Be.FM: Open Foundation Models for Human Behavior
May 29, 2025



Abstract:Despite their success in numerous fields, the potential of foundation models for modeling and understanding human behavior remains largely unexplored. We introduce Be.FM, one of the first open foundation models designed for human behavior modeling. Built upon open-source large language models and fine-tuned on a diverse range of behavioral data, Be.FM can be used to understand and predict human decision-making. We construct a comprehensive set of benchmark tasks for testing the capabilities of behavioral foundation models. Our results demonstrate that Be.FM can predict behaviors, infer characteristics of individuals and populations, generate insights about contexts, and apply behavioral science knowledge.
Interpreting Chest X-rays Like a Radiologist: A Benchmark with Clinical Reasoning
May 29, 2025



Abstract:Artificial intelligence (AI)-based chest X-ray (CXR) interpretation assistants have demonstrated significant progress and are increasingly being applied in clinical settings. However, contemporary medical AI models often adhere to a simplistic input-to-output paradigm, directly processing an image and an instruction to generate a result, where the instructions may be integral to the model's architecture. This approach overlooks the modeling of the inherent diagnostic reasoning in chest X-ray interpretation. Such reasoning is typically sequential, where each interpretive stage considers the images, the current task, and the contextual information from previous stages. This oversight leads to several shortcomings, including misalignment with clinical scenarios, contextless reasoning, and untraceable errors. To fill this gap, we construct CXRTrek, a new multi-stage visual question answering (VQA) dataset for CXR interpretation. The dataset is designed to explicitly simulate the diagnostic reasoning process employed by radiologists in real-world clinical settings for the first time. CXRTrek covers 8 sequential diagnostic stages, comprising 428,966 samples and over 11 million question-answer (Q&A) pairs, with an average of 26.29 Q&A pairs per sample. Building on the CXRTrek dataset, we propose a new vision-language large model (VLLM), CXRTrekNet, specifically designed to incorporate the clinical reasoning flow into the VLLM framework. CXRTrekNet effectively models the dependencies between diagnostic stages and captures reasoning patterns within the radiological context. Trained on our dataset, the model consistently outperforms existing medical VLLMs on the CXRTrek benchmarks and demonstrates superior generalization across multiple tasks on five diverse external datasets. The dataset and model can be found in our repository (https://github.com/guanjinquan/CXRTrek).
TAGS: A Test-Time Generalist-Specialist Framework with Retrieval-Augmented Reasoning and Verification
May 23, 2025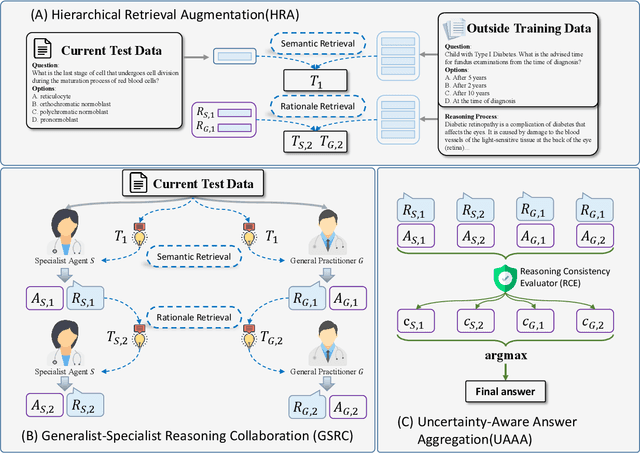
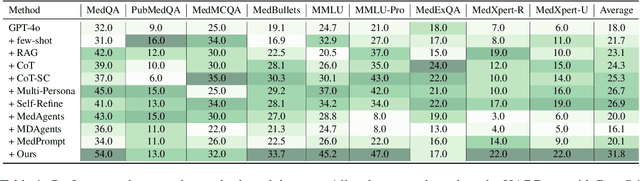
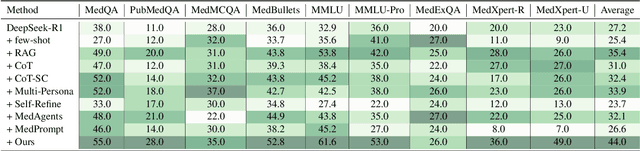
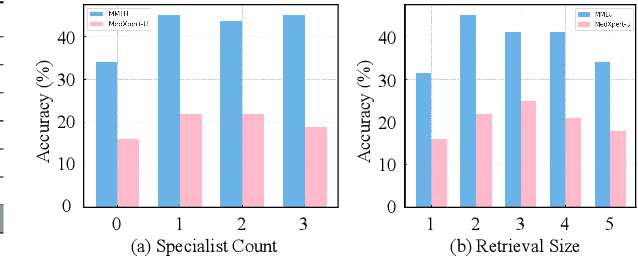
Abstract:Recent advances such as Chain-of-Thought prompting have significantly improved large language models (LLMs) in zero-shot medical reasoning. However, prompting-based methods often remain shallow and unstable, while fine-tuned medical LLMs suffer from poor generalization under distribution shifts and limited adaptability to unseen clinical scenarios. To address these limitations, we present TAGS, a test-time framework that combines a broadly capable generalist with a domain-specific specialist to offer complementary perspectives without any model fine-tuning or parameter updates. To support this generalist-specialist reasoning process, we introduce two auxiliary modules: a hierarchical retrieval mechanism that provides multi-scale exemplars by selecting examples based on both semantic and rationale-level similarity, and a reliability scorer that evaluates reasoning consistency to guide final answer aggregation. TAGS achieves strong performance across nine MedQA benchmarks, boosting GPT-4o accuracy by 13.8%, DeepSeek-R1 by 16.8%, and improving a vanilla 7B model from 14.1% to 23.9%. These results surpass several fine-tuned medical LLMs, without any parameter updates. The code will be available at https://github.com/JianghaoWu/TAGS.
ATR-Bench: A Federated Learning Benchmark for Adaptation, Trust, and Reasoning
May 22, 2025Abstract:Federated Learning (FL) has emerged as a promising paradigm for collaborative model training while preserving data privacy across decentralized participants. As FL adoption grows, numerous techniques have been proposed to tackle its practical challenges. However, the lack of standardized evaluation across key dimensions hampers systematic progress and fair comparison of FL methods. In this work, we introduce ATR-Bench, a unified framework for analyzing federated learning through three foundational dimensions: Adaptation, Trust, and Reasoning. We provide an in-depth examination of the conceptual foundations, task formulations, and open research challenges associated with each theme. We have extensively benchmarked representative methods and datasets for adaptation to heterogeneous clients and trustworthiness in adversarial or unreliable environments. Due to the lack of reliable metrics and models for reasoning in FL, we only provide literature-driven insights for this dimension. ATR-Bench lays the groundwork for a systematic and holistic evaluation of federated learning with real-world relevance. We will make our complete codebase publicly accessible and a curated repository that continuously tracks new developments and research in the FL literature.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge