Peng Xia
SkillRL: Evolving Agents via Recursive Skill-Augmented Reinforcement Learning
Feb 09, 2026Abstract:Large Language Model (LLM) agents have shown stunning results in complex tasks, yet they often operate in isolation, failing to learn from past experiences. Existing memory-based methods primarily store raw trajectories, which are often redundant and noise-heavy. This prevents agents from extracting high-level, reusable behavioral patterns that are essential for generalization. In this paper, we propose SkillRL, a framework that bridges the gap between raw experience and policy improvement through automatic skill discovery and recursive evolution. Our approach introduces an experience-based distillation mechanism to build a hierarchical skill library SkillBank, an adaptive retrieval strategy for general and task-specific heuristics, and a recursive evolution mechanism that allows the skill library to co-evolve with the agent's policy during reinforcement learning. These innovations significantly reduce the token footprint while enhancing reasoning utility. Experimental results on ALFWorld, WebShop and seven search-augmented tasks demonstrate that SkillRL achieves state-of-the-art performance, outperforming strong baselines over 15.3% and maintaining robustness as task complexity increases. Code is available at this https://github.com/aiming-lab/SkillRL.
MedVerse: Efficient and Reliable Medical Reasoning via DAG-Structured Parallel Execution
Feb 07, 2026Abstract:Large language models (LLMs) have demonstrated strong performance and rapid progress in a wide range of medical reasoning tasks. However, their sequential autoregressive decoding forces inherently parallel clinical reasoning, such as differential diagnosis, into a single linear reasoning path, limiting both efficiency and reliability for complex medical problems. To address this, we propose MedVerse, a reasoning framework for complex medical inference that reformulates medical reasoning as a parallelizable directed acyclic graph (DAG) process based on Petri net theory. The framework adopts a full-stack design across data, model architecture, and system execution. For data creation, we introduce the MedVerse Curator, an automated pipeline that synthesizes knowledge-grounded medical reasoning paths and transforms them into Petri net-structured representations. At the architectural level, we propose a topology-aware attention mechanism with adaptive position indices that supports parallel reasoning while preserving logical consistency. Systematically, we develop a customized inference engine that supports parallel execution without additional overhead. Empirical evaluations show that MedVerse improves strong general-purpose LLMs by up to 8.9%. Compared to specialized medical LLMs, MedVerse achieves comparable performance while delivering a 1.3x reduction in inference latency and a 1.7x increase in generation throughput, enabled by its parallel decoding capability.
Reliable and Responsible Foundation Models: A Comprehensive Survey
Feb 04, 2026Abstract:Foundation models, including Large Language Models (LLMs), Multimodal Large Language Models (MLLMs), Image Generative Models (i.e, Text-to-Image Models and Image-Editing Models), and Video Generative Models, have become essential tools with broad applications across various domains such as law, medicine, education, finance, science, and beyond. As these models see increasing real-world deployment, ensuring their reliability and responsibility has become critical for academia, industry, and government. This survey addresses the reliable and responsible development of foundation models. We explore critical issues, including bias and fairness, security and privacy, uncertainty, explainability, and distribution shift. Our research also covers model limitations, such as hallucinations, as well as methods like alignment and Artificial Intelligence-Generated Content (AIGC) detection. For each area, we review the current state of the field and outline concrete future research directions. Additionally, we discuss the intersections between these areas, highlighting their connections and shared challenges. We hope our survey fosters the development of foundation models that are not only powerful but also ethical, trustworthy, reliable, and socially responsible.
SimpleMem: Efficient Lifelong Memory for LLM Agents
Jan 05, 2026Abstract:To support reliable long-term interaction in complex environments, LLM agents require memory systems that efficiently manage historical experiences. Existing approaches either retain full interaction histories via passive context extension, leading to substantial redundancy, or rely on iterative reasoning to filter noise, incurring high token costs. To address this challenge, we introduce SimpleMem, an efficient memory framework based on semantic lossless compression. We propose a three-stage pipeline designed to maximize information density and token utilization: (1) \textit{Semantic Structured Compression}, which applies entropy-aware filtering to distill unstructured interactions into compact, multi-view indexed memory units; (2) \textit{Recursive Memory Consolidation}, an asynchronous process that integrates related units into higher-level abstract representations to reduce redundancy; and (3) \textit{Adaptive Query-Aware Retrieval}, which dynamically adjusts retrieval scope based on query complexity to construct precise context efficiently. Experiments on benchmark datasets show that our method consistently outperforms baseline approaches in accuracy, retrieval efficiency, and inference cost, achieving an average F1 improvement of 26.4% while reducing inference-time token consumption by up to 30-fold, demonstrating a superior balance between performance and efficiency. Code is available at https://github.com/aiming-lab/SimpleMem.
SilverTorch: A Unified Model-based System to Democratize Large-Scale Recommendation on GPUs
Nov 18, 2025Abstract:Serving deep learning based recommendation models (DLRM) at scale is challenging. Existing systems rely on CPU-based ANN indexing and filtering services, suffering from non-negligible costs and forgoing joint optimization opportunities. Such inefficiency makes them difficult to support more complex model architectures, such as learned similarities and multi-task retrieval. In this paper, we propose SilverTorch, a model-based system for serving recommendation models on GPUs. SilverTorch unifies model serving by replacing standalone indexing and filtering services with layers of served models. We propose a Bloom index algorithm on GPUs for feature filtering and a tensor-native fused Int8 ANN kernel on GPUs for nearest neighbor search. We further co-design the ANN search index and filtering index to reduce GPU memory utilization and eliminate unnecessary computation. Benefit from SilverTorch's serving paradigm, we introduce a OverArch scoring layer and a Value Model to aggregate results across multi-tasks. These advancements improve the accuracy for retrieval and enable future studies for serving more complex models. For ranking, SilverTorch's design accelerates item embedding calculation by caching the pre-calculated embeddings inside the serving model. Our evaluation on the industry-scale datasets show that SilverTorch achieves up to 5.6x lower latency and 23.7x higher throughput compared to the state-of-the-art approaches. We also demonstrate that SilverTorch's solution is 13.35x more cost-efficient than CPU-based solution while improving accuracy via serving more complex models. SilverTorch serves over hundreds of models online across major products and recommends contents for billions of daily active users.
Adaptive Ensemble Learning with Gaussian Copula for Load Forecasting
Aug 25, 2025Abstract:Machine learning (ML) is capable of accurate Load Forecasting from complete data. However, there are many uncertainties that affect data collection, leading to sparsity. This article proposed a model called Adaptive Ensemble Learning with Gaussian Copula to deal with sparsity, which contains three modules: data complementation, ML construction, and adaptive ensemble. First, it applies Gaussian Copula to eliminate sparsity. Then, we utilise five ML models to make predictions individually. Finally, it employs adaptive ensemble to get final weighted-sum result. Experiments have demonstrated that our model are robust.
Mimicking the Physicist's Eye:A VLM-centric Approach for Physics Formula Discovery
Aug 24, 2025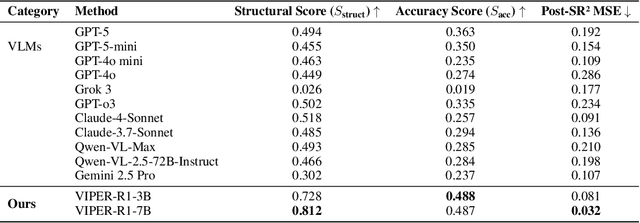
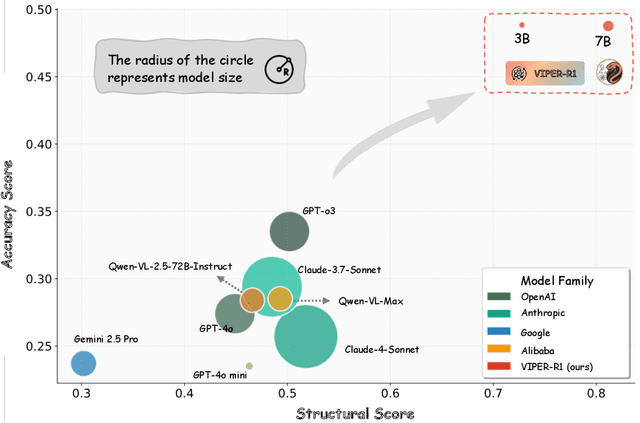
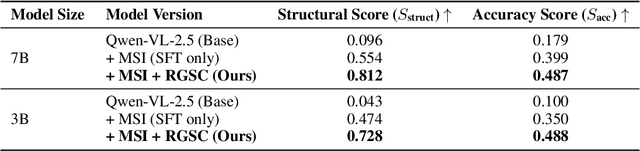
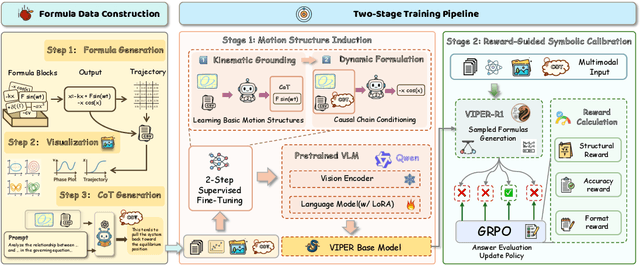
Abstract:Automated discovery of physical laws from observational data in the real world is a grand challenge in AI. Current methods, relying on symbolic regression or LLMs, are limited to uni-modal data and overlook the rich, visual phenomenological representations of motion that are indispensable to physicists. This "sensory deprivation" severely weakens their ability to interpret the inherent spatio-temporal patterns within dynamic phenomena. To address this gap, we propose VIPER-R1, a multimodal model that performs Visual Induction for Physics-based Equation Reasoning to discover fundamental symbolic formulas. It integrates visual perception, trajectory data, and symbolic reasoning to emulate the scientific discovery process. The model is trained via a curriculum of Motion Structure Induction (MSI), using supervised fine-tuning to interpret kinematic phase portraits and to construct hypotheses guided by a Causal Chain of Thought (C-CoT), followed by Reward-Guided Symbolic Calibration (RGSC) to refine the formula structure with reinforcement learning. During inference, the trained VIPER-R1 acts as an agent: it first posits a high-confidence symbolic ansatz, then proactively invokes an external symbolic regression tool to perform Symbolic Residual Realignment (SR^2). This final step, analogous to a physicist's perturbation analysis, reconciles the theoretical model with empirical data. To support this research, we introduce PhysSymbol, a new 5,000-instance multimodal corpus. Experiments show that VIPER-R1 consistently outperforms state-of-the-art VLM baselines in accuracy and interpretability, enabling more precise discovery of physical laws. Project page: https://jiaaqiliu.github.io/VIPER-R1/
Agent KB: Leveraging Cross-Domain Experience for Agentic Problem Solving
Jul 08, 2025Abstract:As language agents tackle increasingly complex tasks, they struggle with effective error correction and experience reuse across domains. We introduce Agent KB, a hierarchical experience framework that enables complex agentic problem solving via a novel Reason-Retrieve-Refine pipeline. Agent KB addresses a core limitation: agents traditionally cannot learn from each other's experiences. By capturing both high-level strategies and detailed execution logs, Agent KB creates a shared knowledge base that enables cross-agent knowledge transfer. Evaluated on the GAIA benchmark, Agent KB improves success rates by up to 16.28 percentage points. On the most challenging tasks, Claude-3 improves from 38.46% to 57.69%, while GPT-4 improves from 53.49% to 73.26% on intermediate tasks. On SWE-bench code repair, Agent KB enables Claude-3 to improve from 41.33% to 53.33%. Our results suggest that Agent KB provides a modular, framework-agnostic infrastructure for enabling agents to learn from past experiences and generalize successful strategies to new tasks.
ChemMLLM: Chemical Multimodal Large Language Model
May 22, 2025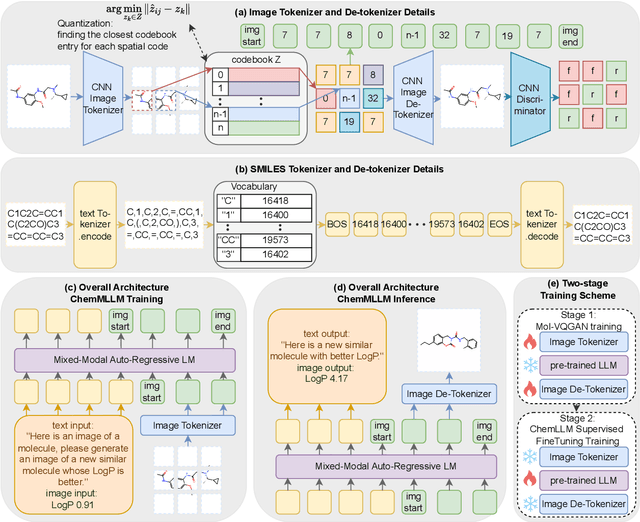
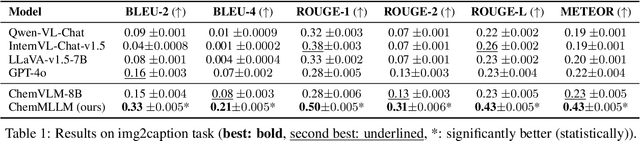
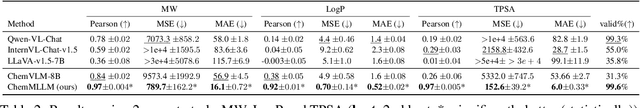
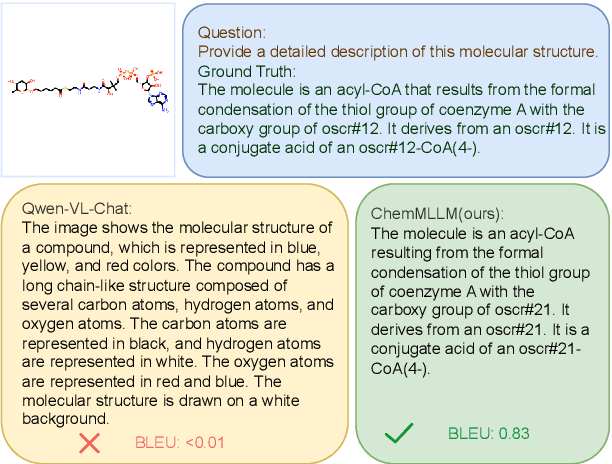
Abstract:Multimodal large language models (MLLMs) have made impressive progress in many applications in recent years. However, chemical MLLMs that can handle cross-modal understanding and generation remain underexplored. To fill this gap, in this paper, we propose ChemMLLM, a unified chemical multimodal large language model for molecule understanding and generation. Also, we design five multimodal tasks across text, molecular SMILES strings, and image, and curate the datasets. We benchmark ChemMLLM against a range of general leading MLLMs and Chemical LLMs on these tasks. Experimental results show that ChemMLLM achieves superior performance across all evaluated tasks. For example, in molecule image optimization task, ChemMLLM outperforms the best baseline (GPT-4o) by 118.9\% (4.27 vs 1.95 property improvement). The code is publicly available at https://github.com/bbsbz/ChemMLLM.git.
Anyprefer: An Agentic Framework for Preference Data Synthesis
Apr 27, 2025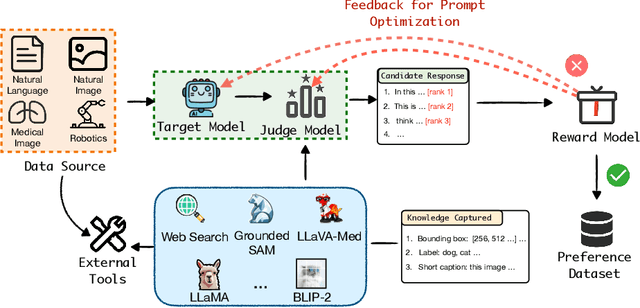
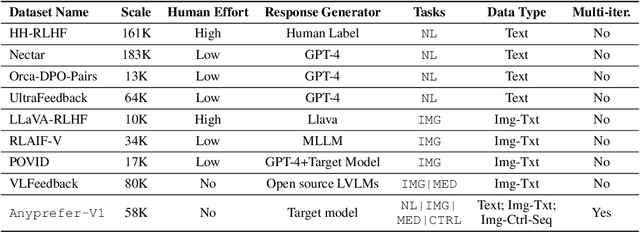
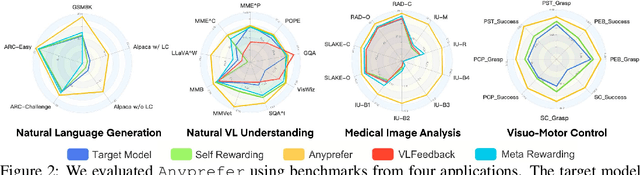
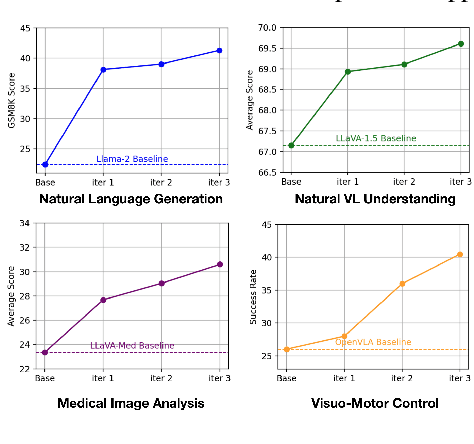
Abstract:High-quality preference data is essential for aligning foundation models with human values through preference learning. However, manual annotation of such data is often time-consuming and costly. Recent methods often adopt a self-rewarding approach, where the target model generates and annotates its own preference data, but this can lead to inaccuracies since the reward model shares weights with the target model, thereby amplifying inherent biases. To address these issues, we propose Anyprefer, a framework designed to synthesize high-quality preference data for aligning the target model. Anyprefer frames the data synthesis process as a cooperative two-player Markov Game, where the target model and the judge model collaborate together. Here, a series of external tools are introduced to assist the judge model in accurately rewarding the target model's responses, mitigating biases in the rewarding process. In addition, a feedback mechanism is introduced to optimize prompts for both models, enhancing collaboration and improving data quality. The synthesized data is compiled into a new preference dataset, Anyprefer-V1, consisting of 58K high-quality preference pairs. Extensive experiments show that Anyprefer significantly improves model alignment performance across four main applications, covering 21 datasets, achieving average improvements of 18.55% in five natural language generation datasets, 3.66% in nine vision-language understanding datasets, 30.05% in three medical image analysis datasets, and 16.00% in four visuo-motor control tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge