Xiang Wan
Eliminating Inductive Bias in Reward Models with Information-Theoretic Guidance
Dec 29, 2025Abstract:Reward models (RMs) are essential in reinforcement learning from human feedback (RLHF) to align large language models (LLMs) with human values. However, RM training data is commonly recognized as low-quality, containing inductive biases that can easily lead to overfitting and reward hacking. For example, more detailed and comprehensive responses are usually human-preferred but with more words, leading response length to become one of the inevitable inductive biases. A limited number of prior RM debiasing approaches either target a single specific type of bias or model the problem with only simple linear correlations, \textit{e.g.}, Pearson coefficients. To mitigate more complex and diverse inductive biases in reward modeling, we introduce a novel information-theoretic debiasing method called \textbf{D}ebiasing via \textbf{I}nformation optimization for \textbf{R}M (DIR). Inspired by the information bottleneck (IB), we maximize the mutual information (MI) between RM scores and human preference pairs, while minimizing the MI between RM outputs and biased attributes of preference inputs. With theoretical justification from information theory, DIR can handle more sophisticated types of biases with non-linear correlations, broadly extending the real-world application scenarios for RM debiasing methods. In experiments, we verify the effectiveness of DIR with three types of inductive biases: \textit{response length}, \textit{sycophancy}, and \textit{format}. We discover that DIR not only effectively mitigates target inductive biases but also enhances RLHF performance across diverse benchmarks, yielding better generalization abilities. The code and training recipes are available at https://github.com/Qwen-Applications/DIR.
DGSAN: Dual-Graph Spatiotemporal Attention Network for Pulmonary Nodule Malignancy Prediction
Dec 24, 2025Abstract:Lung cancer continues to be the leading cause of cancer-related deaths globally. Early detection and diagnosis of pulmonary nodules are essential for improving patient survival rates. Although previous research has integrated multimodal and multi-temporal information, outperforming single modality and single time point, the fusion methods are limited to inefficient vector concatenation and simple mutual attention, highlighting the need for more effective multimodal information fusion. To address these challenges, we introduce a Dual-Graph Spatiotemporal Attention Network, which leverages temporal variations and multimodal data to enhance the accuracy of predictions. Our methodology involves developing a Global-Local Feature Encoder to better capture the local, global, and fused characteristics of pulmonary nodules. Additionally, a Dual-Graph Construction method organizes multimodal features into inter-modal and intra-modal graphs. Furthermore, a Hierarchical Cross-Modal Graph Fusion Module is introduced to refine feature integration. We also compiled a novel multimodal dataset named the NLST-cmst dataset as a comprehensive source of support for related research. Our extensive experiments, conducted on both the NLST-cmst and curated CSTL-derived datasets, demonstrate that our DGSAN significantly outperforms state-of-the-art methods in classifying pulmonary nodules with exceptional computational efficiency.
MedGEN-Bench: Contextually entangled benchmark for open-ended multimodal medical generation
Nov 18, 2025Abstract:As Vision-Language Models (VLMs) increasingly gain traction in medical applications, clinicians are progressively expecting AI systems not only to generate textual diagnoses but also to produce corresponding medical images that integrate seamlessly into authentic clinical workflows. Despite the growing interest, existing medical visual benchmarks present notable limitations. They often rely on ambiguous queries that lack sufficient relevance to image content, oversimplify complex diagnostic reasoning into closed-ended shortcuts, and adopt a text-centric evaluation paradigm that overlooks the importance of image generation capabilities. To address these challenges, we introduce MedGEN-Bench, a comprehensive multimodal benchmark designed to advance medical AI research. MedGEN-Bench comprises 6,422 expert-validated image-text pairs spanning six imaging modalities, 16 clinical tasks, and 28 subtasks. It is structured into three distinct formats: Visual Question Answering, Image Editing, and Contextual Multimodal Generation. What sets MedGEN-Bench apart is its focus on contextually intertwined instructions that necessitate sophisticated cross-modal reasoning and open-ended generative outputs, moving beyond the constraints of multiple-choice formats. To evaluate the performance of existing systems, we employ a novel three-tier assessment framework that integrates pixel-level metrics, semantic text analysis, and expert-guided clinical relevance scoring. Using this framework, we systematically assess 10 compositional frameworks, 3 unified models, and 5 VLMs.
AdaDrive: Self-Adaptive Slow-Fast System for Language-Grounded Autonomous Driving
Nov 09, 2025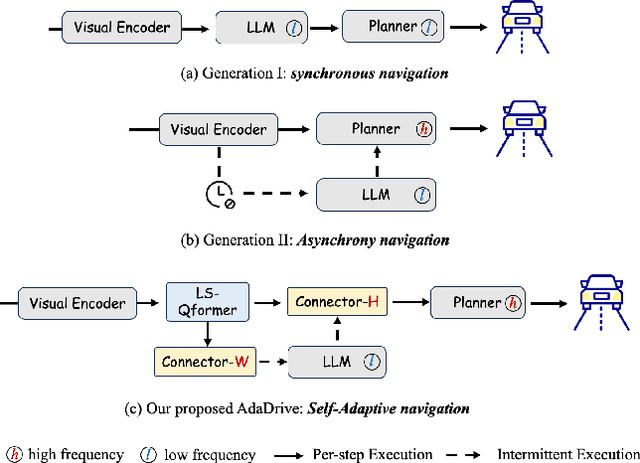
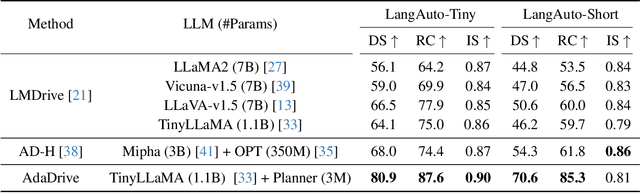
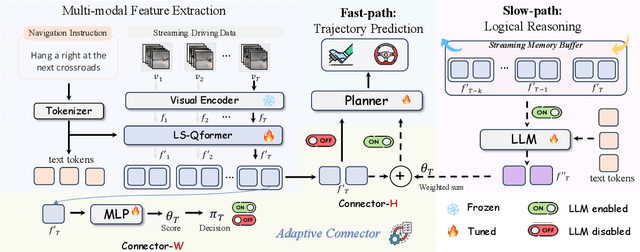
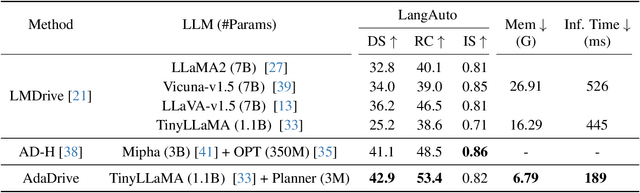
Abstract:Effectively integrating Large Language Models (LLMs) into autonomous driving requires a balance between leveraging high-level reasoning and maintaining real-time efficiency. Existing approaches either activate LLMs too frequently, causing excessive computational overhead, or use fixed schedules, failing to adapt to dynamic driving conditions. To address these challenges, we propose AdaDrive, an adaptively collaborative slow-fast framework that optimally determines when and how LLMs contribute to decision-making. (1) When to activate the LLM: AdaDrive employs a novel adaptive activation loss that dynamically determines LLM invocation based on a comparative learning mechanism, ensuring activation only in complex or critical scenarios. (2) How to integrate LLM assistance: Instead of rigid binary activation, AdaDrive introduces an adaptive fusion strategy that modulates a continuous, scaled LLM influence based on scene complexity and prediction confidence, ensuring seamless collaboration with conventional planners. Through these strategies, AdaDrive provides a flexible, context-aware framework that maximizes decision accuracy without compromising real-time performance. Extensive experiments on language-grounded autonomous driving benchmarks demonstrate that AdaDrive state-of-the-art performance in terms of both driving accuracy and computational efficiency. Code is available at https://github.com/ReaFly/AdaDrive.
VLDrive: Vision-Augmented Lightweight MLLMs for Efficient Language-grounded Autonomous Driving
Nov 09, 2025Abstract:Recent advancements in language-grounded autonomous driving have been significantly promoted by the sophisticated cognition and reasoning capabilities of large language models (LLMs). However, current LLM-based approaches encounter critical challenges: (1) Failure analysis reveals that frequent collisions and obstructions, stemming from limitations in visual representations, remain primary obstacles to robust driving performance. (2) The substantial parameters of LLMs pose considerable deployment hurdles. To address these limitations, we introduce VLDrive, a novel approach featuring a lightweight MLLM architecture with enhanced vision components. VLDrive achieves compact visual tokens through innovative strategies, including cycle-consistent dynamic visual pruning and memory-enhanced feature aggregation. Furthermore, we propose a distance-decoupled instruction attention mechanism to improve joint visual-linguistic feature learning, particularly for long-range visual tokens. Extensive experiments conducted in the CARLA simulator demonstrate VLDrive`s effectiveness. Notably, VLDrive achieves state-of-the-art driving performance while reducing parameters by 81% (from 7B to 1.3B), yielding substantial driving score improvements of 15.4%, 16.8%, and 7.6% at tiny, short, and long distances, respectively, in closed-loop evaluations. Code is available at https://github.com/ReaFly/VLDrive.
ShizhenGPT: Towards Multimodal LLMs for Traditional Chinese Medicine
Aug 20, 2025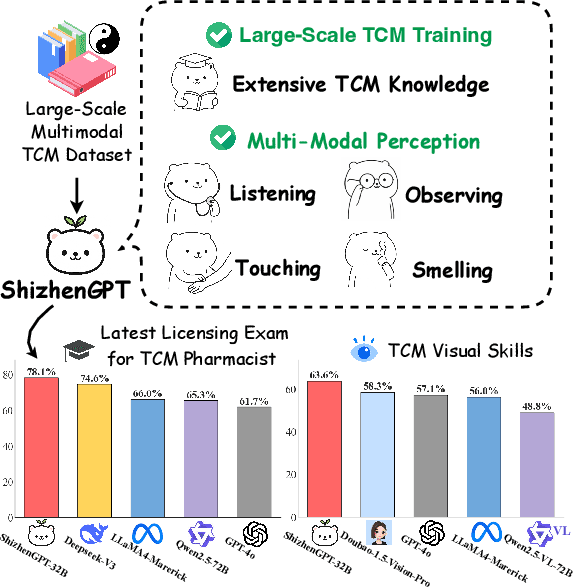
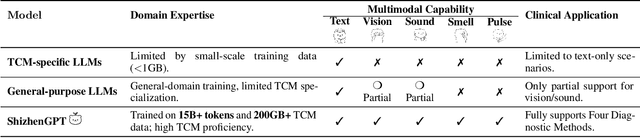
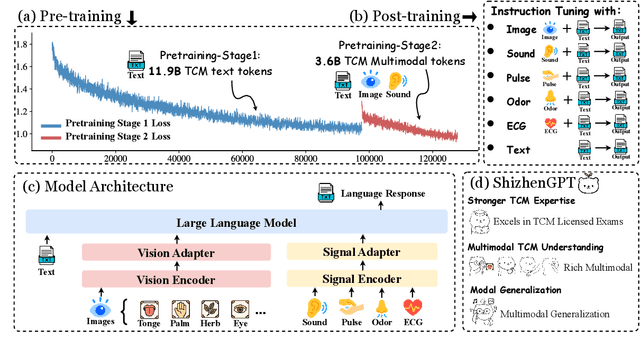
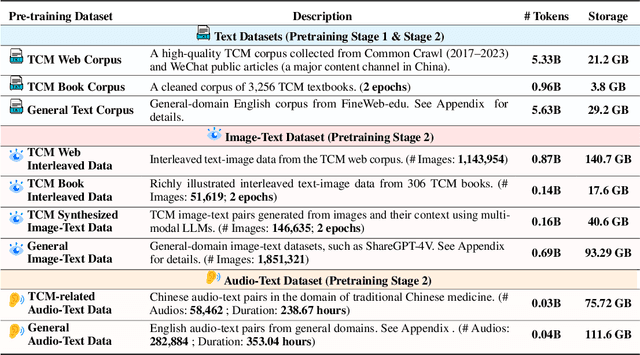
Abstract:Despite the success of large language models (LLMs) in various domains, their potential in Traditional Chinese Medicine (TCM) remains largely underexplored due to two critical barriers: (1) the scarcity of high-quality TCM data and (2) the inherently multimodal nature of TCM diagnostics, which involve looking, listening, smelling, and pulse-taking. These sensory-rich modalities are beyond the scope of conventional LLMs. To address these challenges, we present ShizhenGPT, the first multimodal LLM tailored for TCM. To overcome data scarcity, we curate the largest TCM dataset to date, comprising 100GB+ of text and 200GB+ of multimodal data, including 1.2M images, 200 hours of audio, and physiological signals. ShizhenGPT is pretrained and instruction-tuned to achieve deep TCM knowledge and multimodal reasoning. For evaluation, we collect recent national TCM qualification exams and build a visual benchmark for Medicinal Recognition and Visual Diagnosis. Experiments demonstrate that ShizhenGPT outperforms comparable-scale LLMs and competes with larger proprietary models. Moreover, it leads in TCM visual understanding among existing multimodal LLMs and demonstrates unified perception across modalities like sound, pulse, smell, and vision, paving the way toward holistic multimodal perception and diagnosis in TCM. Datasets, models, and code are publicly available. We hope this work will inspire further exploration in this field.
An Investigation of Robustness of LLMs in Mathematical Reasoning: Benchmarking with Mathematically-Equivalent Transformation of Advanced Mathematical Problems
Aug 12, 2025Abstract:In this paper, we introduce a systematic framework beyond conventional method to assess LLMs' mathematical-reasoning robustness by stress-testing them on advanced math problems that are mathematically equivalent but with linguistic and parametric variation. These transformations allow us to measure the sensitivity of LLMs to non-mathematical perturbations, thereby enabling a more accurate evaluation of their mathematical reasoning capabilities. Using this new evaluation methodology, we created PutnamGAP, a new benchmark dataset with multiple mathematically-equivalent variations of competition-level math problems. With the new dataset, we evaluate multiple families of representative LLMs and examine their robustness. Across 18 commercial and open-source models we observe sharp performance degradation on the variants. OpenAI's flagship reasoning model, O3, scores 49 % on the originals but drops by 4 percentage points on surface variants, and by 10.5 percentage points on core-step-based variants, while smaller models fare far worse. Overall, the results show that the proposed new evaluation methodology is effective for deepening our understanding of the robustness of LLMs and generating new insights for further improving their mathematical reasoning capabilities.
Multi-RIS Deployment Optimization for mmWave ISAC Systems in Real-World Environments
Aug 10, 2025Abstract:Reconfigurable intelligent surface-assisted integrated sensing and communication (RIS-ISAC) presents a promising system architecture to leverage the wide bandwidth available at millimeter-wave (mmWave) frequencies, while mitigating severe signal propagation losses and reducing infrastructure costs. To enhance ISAC functionalities in the future air-ground integrated network applications, RIS deployment must be carefully designed and evaluated, which forms the core motivation of this paper. To ensure practical relevance, a multi-RIS-ISAC system is established, with its signal model at mmWave frequencies demonstrated using ray-launching calibrated to real-world environments. On this basis, an energy-efficiency-driven optimization problem is formulated to minimize the multi-RIS size-to-coverage sum ratio, comprehensively considering real-world RIS deployment constraints, positions, orientations, as well as ISAC beamforming strategies at both the base station and the RISs. To solve the resulting non-convex mixed-integer problem, a simplified reformulation based on equivalent gain scaling method is introduced. A two-step iterative algorithm is then proposed, in which the deployment parameters are determined under fixed RIS positions in the first step, and the RIS position set is updated in the second step to progressively approach the optimum solution. Simulation results based on realistic parameter benchmarks present that the optimized RISs deployment significantly enhances communication coverage and sensing accuracy with the minimum RIS sizes, outperforming existing approaches.
Towards Assessing Medical Ethics from Knowledge to Practice
Aug 07, 2025Abstract:The integration of large language models into healthcare necessitates a rigorous evaluation of their ethical reasoning, an area current benchmarks often overlook. We introduce PrinciplismQA, a comprehensive benchmark with 3,648 questions designed to systematically assess LLMs' alignment with core medical ethics. Grounded in Principlism, our benchmark features a high-quality dataset. This includes multiple-choice questions curated from authoritative textbooks and open-ended questions sourced from authoritative medical ethics case study literature, all validated by medical experts. Our experiments reveal a significant gap between models' ethical knowledge and their practical application, especially in dynamically applying ethical principles to real-world scenarios. Most LLMs struggle with dilemmas concerning Beneficence, often over-emphasizing other principles. Frontier closed-source models, driven by strong general capabilities, currently lead the benchmark. Notably, medical domain fine-tuning can enhance models' overall ethical competence, but further progress requires better alignment with medical ethical knowledge. PrinciplismQA offers a scalable framework to diagnose these specific ethical weaknesses, paving the way for more balanced and responsible medical AI.
Large Language Models for Outpatient Referral: Problem Definition, Benchmarking and Challenges
Mar 11, 2025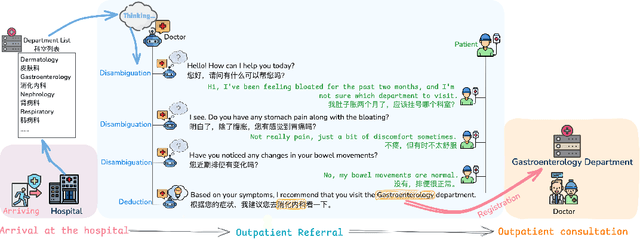
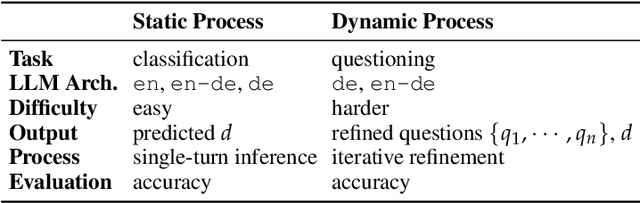
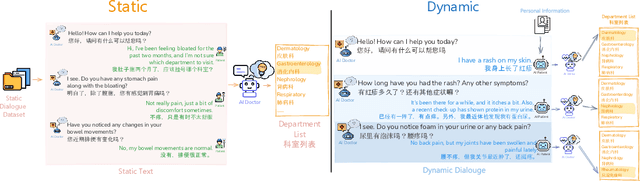
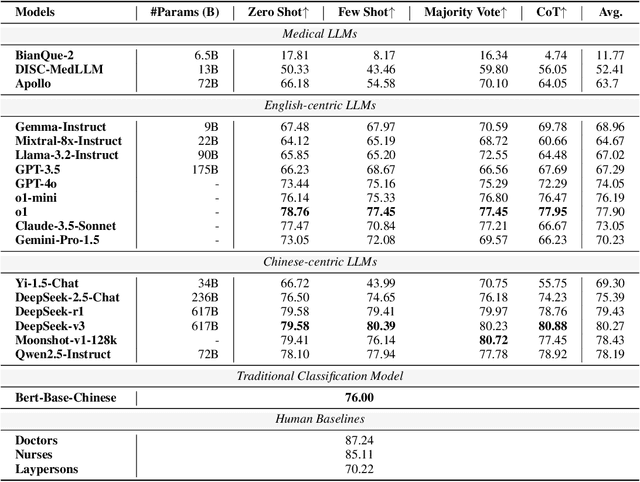
Abstract:Large language models (LLMs) are increasingly applied to outpatient referral tasks across healthcare systems. However, there is a lack of standardized evaluation criteria to assess their effectiveness, particularly in dynamic, interactive scenarios. In this study, we systematically examine the capabilities and limitations of LLMs in managing tasks within Intelligent Outpatient Referral (IOR) systems and propose a comprehensive evaluation framework specifically designed for such systems. This framework comprises two core tasks: static evaluation, which focuses on evaluating the ability of predefined outpatient referrals, and dynamic evaluation, which evaluates capabilities of refining outpatient referral recommendations through iterative dialogues. Our findings suggest that LLMs offer limited advantages over BERT-like models, but show promise in asking effective questions during interactive dialogues.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge