Ruiquan Ge
DGSAN: Dual-Graph Spatiotemporal Attention Network for Pulmonary Nodule Malignancy Prediction
Dec 24, 2025Abstract:Lung cancer continues to be the leading cause of cancer-related deaths globally. Early detection and diagnosis of pulmonary nodules are essential for improving patient survival rates. Although previous research has integrated multimodal and multi-temporal information, outperforming single modality and single time point, the fusion methods are limited to inefficient vector concatenation and simple mutual attention, highlighting the need for more effective multimodal information fusion. To address these challenges, we introduce a Dual-Graph Spatiotemporal Attention Network, which leverages temporal variations and multimodal data to enhance the accuracy of predictions. Our methodology involves developing a Global-Local Feature Encoder to better capture the local, global, and fused characteristics of pulmonary nodules. Additionally, a Dual-Graph Construction method organizes multimodal features into inter-modal and intra-modal graphs. Furthermore, a Hierarchical Cross-Modal Graph Fusion Module is introduced to refine feature integration. We also compiled a novel multimodal dataset named the NLST-cmst dataset as a comprehensive source of support for related research. Our extensive experiments, conducted on both the NLST-cmst and curated CSTL-derived datasets, demonstrate that our DGSAN significantly outperforms state-of-the-art methods in classifying pulmonary nodules with exceptional computational efficiency.
WDT-MD: Wavelet Diffusion Transformers for Microaneurysm Detection in Fundus Images
Nov 16, 2025Abstract:Microaneurysms (MAs), the earliest pathognomonic signs of Diabetic Retinopathy (DR), present as sub-60 $μm$ lesions in fundus images with highly variable photometric and morphological characteristics, rendering manual screening not only labor-intensive but inherently error-prone. While diffusion-based anomaly detection has emerged as a promising approach for automated MA screening, its clinical application is hindered by three fundamental limitations. First, these models often fall prey to "identity mapping", where they inadvertently replicate the input image. Second, they struggle to distinguish MAs from other anomalies, leading to high false positives. Third, their suboptimal reconstruction of normal features hampers overall performance. To address these challenges, we propose a Wavelet Diffusion Transformer framework for MA Detection (WDT-MD), which features three key innovations: a noise-encoded image conditioning mechanism to avoid "identity mapping" by perturbing image conditions during training; pseudo-normal pattern synthesis via inpainting to introduce pixel-level supervision, enabling discrimination between MAs and other anomalies; and a wavelet diffusion Transformer architecture that combines the global modeling capability of diffusion Transformers with multi-scale wavelet analysis to enhance reconstruction of normal retinal features. Comprehensive experiments on the IDRiD and e-ophtha MA datasets demonstrate that WDT-MD outperforms state-of-the-art methods in both pixel-level and image-level MA detection. This advancement holds significant promise for improving early DR screening.
GraphMMP: A Graph Neural Network Model with Mutual Information and Global Fusion for Multimodal Medical Prognosis
Aug 24, 2025Abstract:In the field of multimodal medical data analysis, leveraging diverse types of data and understanding their hidden relationships continues to be a research focus. The main challenges lie in effectively modeling the complex interactions between heterogeneous data modalities with distinct characteristics while capturing both local and global dependencies across modalities. To address these challenges, this paper presents a two-stage multimodal prognosis model, GraphMMP, which is based on graph neural networks. The proposed model constructs feature graphs using mutual information and features a global fusion module built on Mamba, which significantly boosts prognosis performance. Empirical results show that GraphMMP surpasses existing methods on datasets related to liver prognosis and the METABRIC study, demonstrating its effectiveness in multimodal medical prognosis tasks.
Small Lesions-aware Bidirectional Multimodal Multiscale Fusion Network for Lung Disease Classification
Aug 06, 2025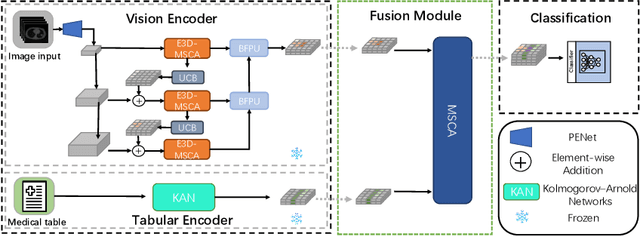
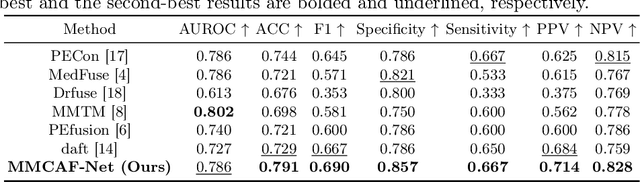
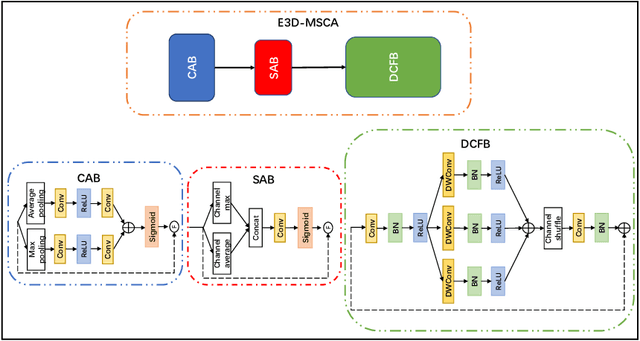
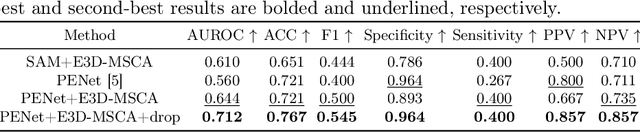
Abstract:The diagnosis of medical diseases faces challenges such as the misdiagnosis of small lesions. Deep learning, particularly multimodal approaches, has shown great potential in the field of medical disease diagnosis. However, the differences in dimensionality between medical imaging and electronic health record data present challenges for effective alignment and fusion. To address these issues, we propose the Multimodal Multiscale Cross-Attention Fusion Network (MMCAF-Net). This model employs a feature pyramid structure combined with an efficient 3D multi-scale convolutional attention module to extract lesion-specific features from 3D medical images. To further enhance multimodal data integration, MMCAF-Net incorporates a multi-scale cross-attention module, which resolves dimensional inconsistencies, enabling more effective feature fusion. We evaluated MMCAF-Net on the Lung-PET-CT-Dx dataset, and the results showed a significant improvement in diagnostic accuracy, surpassing current state-of-the-art methods. The code is available at https://github.com/yjx1234/MMCAF-Net
ITCFN: Incomplete Triple-Modal Co-Attention Fusion Network for Mild Cognitive Impairment Conversion Prediction
Jan 20, 2025


Abstract:Alzheimer's disease (AD) is a common neurodegenerative disease among the elderly. Early prediction and timely intervention of its prodromal stage, mild cognitive impairment (MCI), can decrease the risk of advancing to AD. Combining information from various modalities can significantly improve predictive accuracy. However, challenges such as missing data and heterogeneity across modalities complicate multimodal learning methods as adding more modalities can worsen these issues. Current multimodal fusion techniques often fail to adapt to the complexity of medical data, hindering the ability to identify relationships between modalities. To address these challenges, we propose an innovative multimodal approach for predicting MCI conversion, focusing specifically on the issues of missing positron emission tomography (PET) data and integrating diverse medical information. The proposed incomplete triple-modal MCI conversion prediction network is tailored for this purpose. Through the missing modal generation module, we synthesize the missing PET data from the magnetic resonance imaging and extract features using specifically designed encoders. We also develop a channel aggregation module and a triple-modal co-attention fusion module to reduce feature redundancy and achieve effective multimodal data fusion. Furthermore, we design a loss function to handle missing modality issues and align cross-modal features. These components collectively harness multimodal data to boost network performance. Experimental results on the ADNI1 and ADNI2 datasets show that our method significantly surpasses existing unimodal and other multimodal models. Our code is available at https://github.com/justinhxy/ITFC.
BS-LDM: Effective Bone Suppression in High-Resolution Chest X-Ray Images with Conditional Latent Diffusion Models
Dec 24, 2024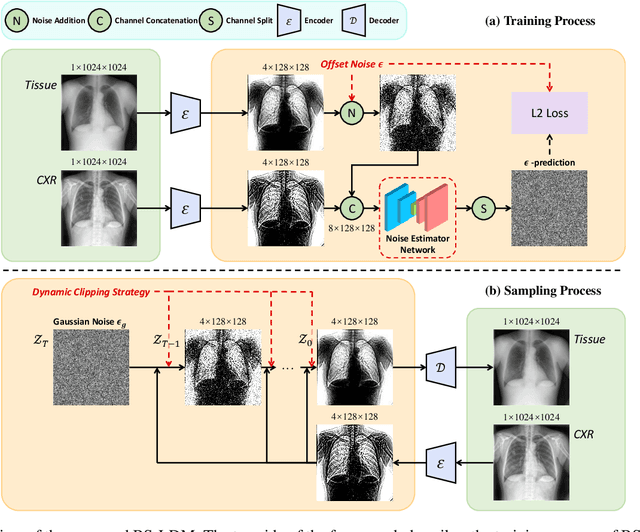
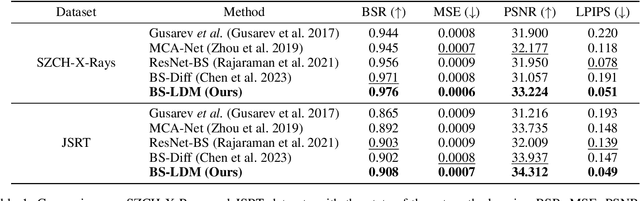
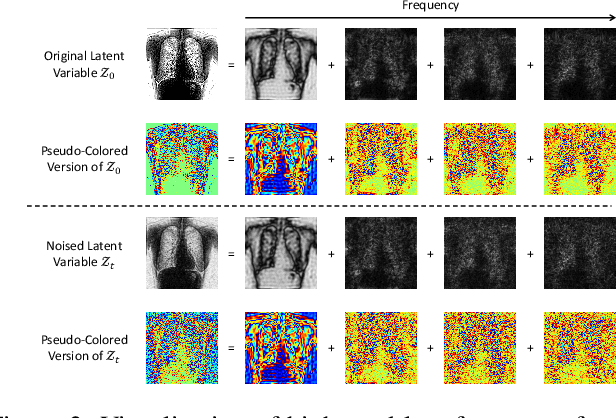
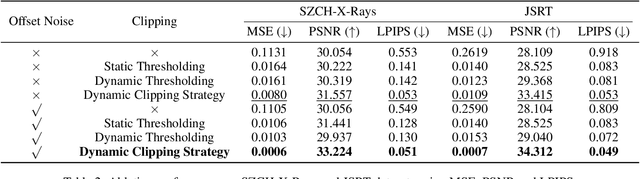
Abstract:The interference of overlapping bones and pulmonary structures can reduce the effectiveness of Chest X-ray (CXR) examinations. Bone suppression techniques have been developed to improve diagnostic accuracy. Dual-energy subtraction (DES) imaging, a common method for bone suppression, is costly and exposes patients to higher radiation levels. Deep learning-based image generation methods have been proposed as alternatives, however, they often fail to produce high-quality and high-resolution images, resulting in the loss of critical lesion information and texture details. To address these issues, in this paper, we introduce an end-to-end framework for bone suppression in high-resolution CXR images, termed BS-LDM. This framework employs a conditional latent diffusion model to generate high-resolution soft tissue images with fine detail and critical lung pathology by performing bone suppression in the latent space. We implement offset noise during the noise addition phase of the training process to better render low-frequency information in soft tissue images. Additionally, we introduce a dynamic clipping strategy during the sampling process to refine pixel intensity in the generated soft tissue images. We compiled a substantial and high-quality bone suppression dataset, SZCH-X-Rays, including high-resolution paired CXR and DES soft tissue images from 818 patients, collected from our partner hospitals. Moreover, we pre-processed 241 pairs of CXR and DES soft tissue images from the JSRT dataset, the largest publicly available dataset. Comprehensive experimental and clinical evaluations demonstrate that BS-LDM exhibits superior bone suppression capabilities, highlighting its significant clinical potential.
ICH-SCNet: Intracerebral Hemorrhage Segmentation and Prognosis Classification Network Using CLIP-guided SAM mechanism
Nov 07, 2024Abstract:Intracerebral hemorrhage (ICH) is the most fatal subtype of stroke and is characterized by a high incidence of disability. Accurate segmentation of the ICH region and prognosis prediction are critically important for developing and refining treatment plans for post-ICH patients. However, existing approaches address these two tasks independently and predominantly focus on imaging data alone, thereby neglecting the intrinsic correlation between the tasks and modalities. This paper introduces a multi-task network, ICH-SCNet, designed for both ICH segmentation and prognosis classification. Specifically, we integrate a SAM-CLIP cross-modal interaction mechanism that combines medical text and segmentation auxiliary information with neuroimaging data to enhance cross-modal feature recognition. Additionally, we develop an effective feature fusion module and a multi-task loss function to improve performance further. Extensive experiments on an ICH dataset reveal that our approach surpasses other state-of-the-art methods. It excels in the overall performance of classification tasks and outperforms competing models in all segmentation task metrics.
LPUWF-LDM: Enhanced Latent Diffusion Model for Precise Late-phase UWF-FA Generation on Limited Dataset
Sep 01, 2024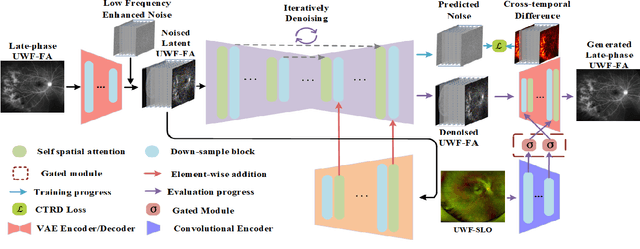
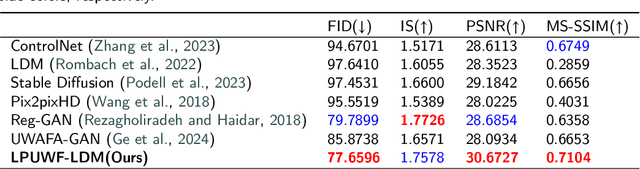
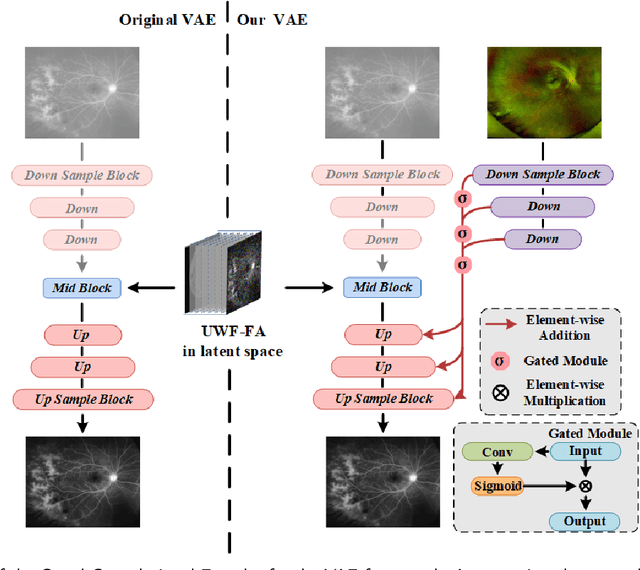

Abstract:Ultra-Wide-Field Fluorescein Angiography (UWF-FA) enables precise identification of ocular diseases using sodium fluorescein, which can be potentially harmful. Existing research has developed methods to generate UWF-FA from Ultra-Wide-Field Scanning Laser Ophthalmoscopy (UWF-SLO) to reduce the adverse reactions associated with injections. However, these methods have been less effective in producing high-quality late-phase UWF-FA, particularly in lesion areas and fine details. Two primary challenges hinder the generation of high-quality late-phase UWF-FA: the scarcity of paired UWF-SLO and early/late-phase UWF-FA datasets, and the need for realistic generation at lesion sites and potential blood leakage regions. This study introduces an improved latent diffusion model framework to generate high-quality late-phase UWF-FA from limited paired UWF images. To address the challenges as mentioned earlier, our approach employs a module utilizing Cross-temporal Regional Difference Loss, which encourages the model to focus on the differences between early and late phases. Additionally, we introduce a low-frequency enhanced noise strategy in the diffusion forward process to improve the realism of medical images. To further enhance the mapping capability of the variational autoencoder module, especially with limited datasets, we implement a Gated Convolutional Encoder to extract additional information from conditional images. Our Latent Diffusion Model for Ultra-Wide-Field Late-Phase Fluorescein Angiography (LPUWF-LDM) effectively reconstructs fine details in late-phase UWF-FA and achieves state-of-the-art results compared to other existing methods when working with limited datasets. Our source code is available at: https://github.com/Tinysqua/****.
TC-KANRecon: High-Quality and Accelerated MRI Reconstruction via Adaptive KAN Mechanisms and Intelligent Feature Scaling
Aug 11, 2024



Abstract:Magnetic Resonance Imaging (MRI) has become essential in clinical diagnosis due to its high resolution and multiple contrast mechanisms. However, the relatively long acquisition time limits its broader application. To address this issue, this study presents an innovative conditional guided diffusion model, named as TC-KANRecon, which incorporates the Multi-Free U-KAN (MF-UKAN) module and a dynamic clipping strategy. TC-KANRecon model aims to accelerate the MRI reconstruction process through deep learning methods while maintaining the quality of the reconstructed images. The MF-UKAN module can effectively balance the tradeoff between image denoising and structure preservation. Specifically, it presents the multi-head attention mechanisms and scalar modulation factors, which significantly enhances the model's robustness and structure preservation capabilities in complex noise environments. Moreover, the dynamic clipping strategy in TC-KANRecon adjusts the cropping interval according to the sampling steps, thereby mitigating image detail loss typically caused by traditional cropping methods and enriching the visual features of the images. Furthermore, the MC-Model module incorporates full-sampling k-space information, realizing efficient fusion of conditional information, enhancing the model's ability to process complex data, and improving the realism and detail richness of reconstructed images. Experimental results demonstrate that the proposed method outperforms other MRI reconstruction methods in both qualitative and quantitative evaluations. Notably, TC-KANRecon method exhibits excellent reconstruction results when processing high-noise, low-sampling-rate MRI data. Our source code is available at https://github.com/lcbkmm/TC-KANRecon.
UWAFA-GAN: Ultra-Wide-Angle Fluorescein Angiography Transformation via Multi-scale Generation and Registration Enhancement
May 01, 2024



Abstract:Fundus photography, in combination with the ultra-wide-angle fundus (UWF) techniques, becomes an indispensable diagnostic tool in clinical settings by offering a more comprehensive view of the retina. Nonetheless, UWF fluorescein angiography (UWF-FA) necessitates the administration of a fluorescent dye via injection into the patient's hand or elbow unlike UWF scanning laser ophthalmoscopy (UWF-SLO). To mitigate potential adverse effects associated with injections, researchers have proposed the development of cross-modality medical image generation algorithms capable of converting UWF-SLO images into their UWF-FA counterparts. Current image generation techniques applied to fundus photography encounter difficulties in producing high-resolution retinal images, particularly in capturing minute vascular lesions. To address these issues, we introduce a novel conditional generative adversarial network (UWAFA-GAN) to synthesize UWF-FA from UWF-SLO. This approach employs multi-scale generators and an attention transmit module to efficiently extract both global structures and local lesions. Additionally, to counteract the image blurriness issue that arises from training with misaligned data, a registration module is integrated within this framework. Our method performs non-trivially on inception scores and details generation. Clinical user studies further indicate that the UWF-FA images generated by UWAFA-GAN are clinically comparable to authentic images in terms of diagnostic reliability. Empirical evaluations on our proprietary UWF image datasets elucidate that UWAFA-GAN outperforms extant methodologies. The code is accessible at https://github.com/Tinysqua/UWAFA-GAN.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge