Feiwei Qin
AnyAD: Unified Any-Modality Anomaly Detection in Incomplete Multi-Sequence MRI
Dec 24, 2025Abstract:Reliable anomaly detection in brain MRI remains challenging due to the scarcity of annotated abnormal cases and the frequent absence of key imaging modalities in real clinical workflows. Existing single-class or multi-class anomaly detection (AD) models typically rely on fixed modality configurations, require repetitive training, or fail to generalize to unseen modality combinations, limiting their clinical scalability. In this work, we present a unified Any-Modality AD framework that performs robust anomaly detection and localization under arbitrary MRI modality availability. The framework integrates a dual-pathway DINOv2 encoder with a feature distribution alignment mechanism that statistically aligns incomplete-modality features with full-modality representations, enabling stable inference even with severe modality dropout. To further enhance semantic consistency, we introduce an Intrinsic Normal Prototypes (INPs) extractor and an INP-guided decoder that reconstruct only normal anatomical patterns while naturally amplifying abnormal deviations. Through randomized modality masking and indirect feature completion during training, the model learns to adapt to all modality configurations without re-training. Extensive experiments on BraTS2018, MU-Glioma-Post, and Pretreat-MetsToBrain-Masks demonstrate that our approach consistently surpasses state-of-the-art industrial and medical AD baselines across 7 modality combinations, achieving superior generalization. This study establishes a scalable paradigm for multimodal medical AD under real-world, imperfect modality conditions. Our source code is available at https://github.com/wuchangw/AnyAD.
MedGEN-Bench: Contextually entangled benchmark for open-ended multimodal medical generation
Nov 18, 2025Abstract:As Vision-Language Models (VLMs) increasingly gain traction in medical applications, clinicians are progressively expecting AI systems not only to generate textual diagnoses but also to produce corresponding medical images that integrate seamlessly into authentic clinical workflows. Despite the growing interest, existing medical visual benchmarks present notable limitations. They often rely on ambiguous queries that lack sufficient relevance to image content, oversimplify complex diagnostic reasoning into closed-ended shortcuts, and adopt a text-centric evaluation paradigm that overlooks the importance of image generation capabilities. To address these challenges, we introduce MedGEN-Bench, a comprehensive multimodal benchmark designed to advance medical AI research. MedGEN-Bench comprises 6,422 expert-validated image-text pairs spanning six imaging modalities, 16 clinical tasks, and 28 subtasks. It is structured into three distinct formats: Visual Question Answering, Image Editing, and Contextual Multimodal Generation. What sets MedGEN-Bench apart is its focus on contextually intertwined instructions that necessitate sophisticated cross-modal reasoning and open-ended generative outputs, moving beyond the constraints of multiple-choice formats. To evaluate the performance of existing systems, we employ a novel three-tier assessment framework that integrates pixel-level metrics, semantic text analysis, and expert-guided clinical relevance scoring. Using this framework, we systematically assess 10 compositional frameworks, 3 unified models, and 5 VLMs.
Brain-HGCN: A Hyperbolic Graph Convolutional Network for Brain Functional Network Analysis
Sep 18, 2025Abstract:Functional magnetic resonance imaging (fMRI) provides a powerful non-invasive window into the brain's functional organization by generating complex functional networks, typically modeled as graphs. These brain networks exhibit a hierarchical topology that is crucial for cognitive processing. However, due to inherent spatial constraints, standard Euclidean GNNs struggle to represent these hierarchical structures without high distortion, limiting their clinical performance. To address this limitation, we propose Brain-HGCN, a geometric deep learning framework based on hyperbolic geometry, which leverages the intrinsic property of negatively curved space to model the brain's network hierarchy with high fidelity. Grounded in the Lorentz model, our model employs a novel hyperbolic graph attention layer with a signed aggregation mechanism to distinctly process excitatory and inhibitory connections, ultimately learning robust graph-level representations via a geometrically sound Fr\'echet mean for graph readout. Experiments on two large-scale fMRI datasets for psychiatric disorder classification demonstrate that our approach significantly outperforms a wide range of state-of-the-art Euclidean baselines. This work pioneers a new geometric deep learning paradigm for fMRI analysis, highlighting the immense potential of hyperbolic GNNs in the field of computational psychiatry.
No Modality Left Behind: Adapting to Missing Modalities via Knowledge Distillation for Brain Tumor Segmentation
Sep 18, 2025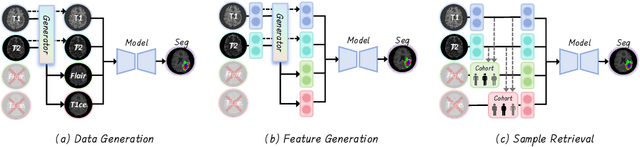
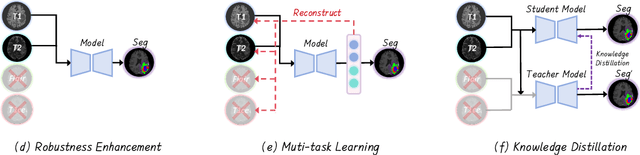
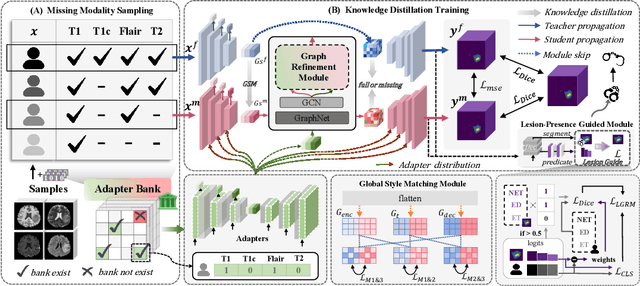
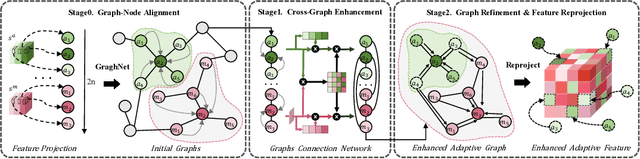
Abstract:Accurate brain tumor segmentation is essential for preoperative evaluation and personalized treatment. Multi-modal MRI is widely used due to its ability to capture complementary tumor features across different sequences. However, in clinical practice, missing modalities are common, limiting the robustness and generalizability of existing deep learning methods that rely on complete inputs, especially under non-dominant modality combinations. To address this, we propose AdaMM, a multi-modal brain tumor segmentation framework tailored for missing-modality scenarios, centered on knowledge distillation and composed of three synergistic modules. The Graph-guided Adaptive Refinement Module explicitly models semantic associations between generalizable and modality-specific features, enhancing adaptability to modality absence. The Bi-Bottleneck Distillation Module transfers structural and textural knowledge from teacher to student models via global style matching and adversarial feature alignment. The Lesion-Presence-Guided Reliability Module predicts prior probabilities of lesion types through an auxiliary classification task, effectively suppressing false positives under incomplete inputs. Extensive experiments on the BraTS 2018 and 2024 datasets demonstrate that AdaMM consistently outperforms existing methods, exhibiting superior segmentation accuracy and robustness, particularly in single-modality and weak-modality configurations. In addition, we conduct a systematic evaluation of six categories of missing-modality strategies, confirming the superiority of knowledge distillation and offering practical guidance for method selection and future research. Our source code is available at https://github.com/Quanato607/AdaMM.
Drawing2CAD: Sequence-to-Sequence Learning for CAD Generation from Vectorized Drawings
Aug 26, 2025



Abstract:Computer-Aided Design (CAD) generative modeling is driving significant innovations across industrial applications. Recent works have shown remarkable progress in creating solid models from various inputs such as point clouds, meshes, and text descriptions. However, these methods fundamentally diverge from traditional industrial workflows that begin with 2D engineering drawings. The automatic generation of parametric CAD models from these 2D vector drawings remains underexplored despite being a critical step in engineering design. To address this gap, our key insight is to reframe CAD generation as a sequence-to-sequence learning problem where vector drawing primitives directly inform the generation of parametric CAD operations, preserving geometric precision and design intent throughout the transformation process. We propose Drawing2CAD, a framework with three key technical components: a network-friendly vector primitive representation that preserves precise geometric information, a dual-decoder transformer architecture that decouples command type and parameter generation while maintaining precise correspondence, and a soft target distribution loss function accommodating inherent flexibility in CAD parameters. To train and evaluate Drawing2CAD, we create CAD-VGDrawing, a dataset of paired engineering drawings and parametric CAD models, and conduct thorough experiments to demonstrate the effectiveness of our method. Code and dataset are available at https://github.com/lllssc/Drawing2CAD.
Bridging the Gap in Missing Modalities: Leveraging Knowledge Distillation and Style Matching for Brain Tumor Segmentation
Jul 30, 2025Abstract:Accurate and reliable brain tumor segmentation, particularly when dealing with missing modalities, remains a critical challenge in medical image analysis. Previous studies have not fully resolved the challenges of tumor boundary segmentation insensitivity and feature transfer in the absence of key imaging modalities. In this study, we introduce MST-KDNet, aimed at addressing these critical issues. Our model features Multi-Scale Transformer Knowledge Distillation to effectively capture attention weights at various resolutions, Dual-Mode Logit Distillation to improve the transfer of knowledge, and a Global Style Matching Module that integrates feature matching with adversarial learning. Comprehensive experiments conducted on the BraTS and FeTS 2024 datasets demonstrate that MST-KDNet surpasses current leading methods in both Dice and HD95 scores, particularly in conditions with substantial modality loss. Our approach shows exceptional robustness and generalization potential, making it a promising candidate for real-world clinical applications. Our source code is available at https://github.com/Quanato607/MST-KDNet.
* 11 pages, 2 figures
Towards Practical Alzheimer's Disease Diagnosis: A Lightweight and Interpretable Spiking Neural Model
Jun 11, 2025Abstract:Early diagnosis of Alzheimer's Disease (AD), especially at the mild cognitive impairment (MCI) stage, is vital yet hindered by subjective assessments and the high cost of multimodal imaging modalities. Although deep learning methods offer automated alternatives, their energy inefficiency and computational demands limit real-world deployment, particularly in resource-constrained settings. As a brain-inspired paradigm, spiking neural networks (SNNs) are inherently well-suited for modeling the sparse, event-driven patterns of neural degeneration in AD, offering a promising foundation for interpretable and low-power medical diagnostics. However, existing SNNs often suffer from weak expressiveness and unstable training, which restrict their effectiveness in complex medical tasks. To address these limitations, we propose FasterSNN, a hybrid neural architecture that integrates biologically inspired LIF neurons with region-adaptive convolution and multi-scale spiking attention. This design enables sparse, efficient processing of 3D MRI while preserving diagnostic accuracy. Experiments on benchmark datasets demonstrate that FasterSNN achieves competitive performance with substantially improved efficiency and stability, supporting its potential for practical AD screening. Our source code is available at https://github.com/wuchangw/FasterSNN.
Towards Accurate and Interpretable Neuroblastoma Diagnosis via Contrastive Multi-scale Pathological Image Analysis
Apr 18, 2025



Abstract:Neuroblastoma, adrenal-derived, is among the most common pediatric solid malignancies, characterized by significant clinical heterogeneity. Timely and accurate pathological diagnosis from hematoxylin and eosin-stained whole slide images is critical for patient prognosis. However, current diagnostic practices primarily rely on subjective manual examination by pathologists, leading to inconsistent accuracy. Existing automated whole slide image classification methods encounter challenges such as poor interpretability, limited feature extraction capabilities, and high computational costs, restricting their practical clinical deployment. To overcome these limitations, we propose CMSwinKAN, a contrastive-learning-based multi-scale feature fusion model tailored for pathological image classification, which enhances the Swin Transformer architecture by integrating a Kernel Activation Network within its multilayer perceptron and classification head modules, significantly improving both interpretability and accuracy. By fusing multi-scale features and leveraging contrastive learning strategies, CMSwinKAN mimics clinicians' comprehensive approach, effectively capturing global and local tissue characteristics. Additionally, we introduce a heuristic soft voting mechanism guided by clinical insights to seamlessly bridge patch-level predictions to whole slide image-level classifications. We validate CMSwinKAN on the PpNTs dataset, which was collaboratively established with our partner hospital and the publicly accessible BreakHis dataset. Results demonstrate that CMSwinKAN performs better than existing state-of-the-art pathology-specific models pre-trained on large datasets. Our source code is available at https://github.com/JSLiam94/CMSwinKAN.
MMLNB: Multi-Modal Learning for Neuroblastoma Subtyping Classification Assisted with Textual Description Generation
Mar 17, 2025Abstract:Neuroblastoma (NB), a leading cause of childhood cancer mortality, exhibits significant histopathological variability, necessitating precise subtyping for accurate prognosis and treatment. Traditional diagnostic methods rely on subjective evaluations that are time-consuming and inconsistent. To address these challenges, we introduce MMLNB, a multi-modal learning (MML) model that integrates pathological images with generated textual descriptions to improve classification accuracy and interpretability. The approach follows a two-stage process. First, we fine-tune a Vision-Language Model (VLM) to enhance pathology-aware text generation. Second, the fine-tuned VLM generates textual descriptions, using a dual-branch architecture to independently extract visual and textual features. These features are fused via Progressive Robust Multi-Modal Fusion (PRMF) Block for stable training. Experimental results show that the MMLNB model is more accurate than the single modal model. Ablation studies demonstrate the importance of multi-modal fusion, fine-tuning, and the PRMF mechanism. This research creates a scalable AI-driven framework for digital pathology, enhancing reliability and interpretability in NB subtyping classification. Our source code is available at https://github.com/HovChen/MMLNB.
XLSTM-HVED: Cross-Modal Brain Tumor Segmentation and MRI Reconstruction Method Using Vision XLSTM and Heteromodal Variational Encoder-Decoder
Dec 09, 2024



Abstract:Neurogliomas are among the most aggressive forms of cancer, presenting considerable challenges in both treatment and monitoring due to their unpredictable biological behavior. Magnetic resonance imaging (MRI) is currently the preferred method for diagnosing and monitoring gliomas. However, the lack of specific imaging techniques often compromises the accuracy of tumor segmentation during the imaging process. To address this issue, we introduce the XLSTM-HVED model. This model integrates a hetero-modal encoder-decoder framework with the Vision XLSTM module to reconstruct missing MRI modalities. By deeply fusing spatial and temporal features, it enhances tumor segmentation performance. The key innovation of our approach is the Self-Attention Variational Encoder (SAVE) module, which improves the integration of modal features. Additionally, it optimizes the interaction of features between segmentation and reconstruction tasks through the Squeeze-Fusion-Excitation Cross Awareness (SFECA) module. Our experiments using the BraTS 2024 dataset demonstrate that our model significantly outperforms existing advanced methods in handling cases where modalities are missing. Our source code is available at https://github.com/Quanato607/XLSTM-HVED.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge