Jin Fan
Towards Practical Alzheimer's Disease Diagnosis: A Lightweight and Interpretable Spiking Neural Model
Jun 11, 2025Abstract:Early diagnosis of Alzheimer's Disease (AD), especially at the mild cognitive impairment (MCI) stage, is vital yet hindered by subjective assessments and the high cost of multimodal imaging modalities. Although deep learning methods offer automated alternatives, their energy inefficiency and computational demands limit real-world deployment, particularly in resource-constrained settings. As a brain-inspired paradigm, spiking neural networks (SNNs) are inherently well-suited for modeling the sparse, event-driven patterns of neural degeneration in AD, offering a promising foundation for interpretable and low-power medical diagnostics. However, existing SNNs often suffer from weak expressiveness and unstable training, which restrict their effectiveness in complex medical tasks. To address these limitations, we propose FasterSNN, a hybrid neural architecture that integrates biologically inspired LIF neurons with region-adaptive convolution and multi-scale spiking attention. This design enables sparse, efficient processing of 3D MRI while preserving diagnostic accuracy. Experiments on benchmark datasets demonstrate that FasterSNN achieves competitive performance with substantially improved efficiency and stability, supporting its potential for practical AD screening. Our source code is available at https://github.com/wuchangw/FasterSNN.
Toward Robust Early Detection of Alzheimer's Disease via an Integrated Multimodal Learning Approach
Aug 29, 2024


Abstract:Alzheimer's Disease (AD) is a complex neurodegenerative disorder marked by memory loss, executive dysfunction, and personality changes. Early diagnosis is challenging due to subtle symptoms and varied presentations, often leading to misdiagnosis with traditional unimodal diagnostic methods due to their limited scope. This study introduces an advanced multimodal classification model that integrates clinical, cognitive, neuroimaging, and EEG data to enhance diagnostic accuracy. The model incorporates a feature tagger with a tabular data coding architecture and utilizes the TimesBlock module to capture intricate temporal patterns in Electroencephalograms (EEG) data. By employing Cross-modal Attention Aggregation module, the model effectively fuses Magnetic Resonance Imaging (MRI) spatial information with EEG temporal data, significantly improving the distinction between AD, Mild Cognitive Impairment, and Normal Cognition. Simultaneously, we have constructed the first AD classification dataset that includes three modalities: EEG, MRI, and tabular data. Our innovative approach aims to facilitate early diagnosis and intervention, potentially slowing the progression of AD. The source code and our private ADMC dataset are available at https://github.com/JustlfC03/MSTNet.
GFE-Mamba: Mamba-based AD Multi-modal Progression Assessment via Generative Feature Extraction from MCI
Jul 22, 2024Abstract:Alzheimer's Disease (AD) is an irreversible neurodegenerative disorder that often progresses from Mild Cognitive Impairment (MCI), leading to memory loss and significantly impacting patients' lives. Clinical trials indicate that early targeted interventions for MCI patients can potentially slow or halt the development and progression of AD. Previous research has shown that accurate medical classification requires the inclusion of extensive multimodal data, such as assessment scales and various neuroimaging techniques like Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET). However, consistently tracking the diagnosis of the same individual over time and simultaneously collecting multimodal data poses significant challenges. To address this issue, we introduce GFE-Mamba, a classifier based on Generative Feature Extraction (GFE). This classifier effectively integrates data from assessment scales, MRI, and PET, enabling deeper multimodal fusion. It efficiently extracts both long and short sequence information and incorporates additional information beyond the pixel space. This approach not only improves classification accuracy but also enhances the interpretability and stability of the model. We constructed datasets of over 3000 samples based on the Alzheimer's Disease Neuroimaging Initiative (ADNI) for a two-step training process. Our experimental results demonstrate that the GFE-Mamba model is effective in predicting the conversion from MCI to AD and outperforms several state-of-the-art methods. Our source code and ADNI dataset processing code are available at https://github.com/Tinysqua/GFE-Mamba.
SCKansformer: Fine-Grained Classification of Bone Marrow Cells via Kansformer Backbone and Hierarchical Attention Mechanisms
Jun 14, 2024



Abstract:The incidence and mortality rates of malignant tumors, such as acute leukemia, have risen significantly. Clinically, hospitals rely on cytological examination of peripheral blood and bone marrow smears to diagnose malignant tumors, with accurate blood cell counting being crucial. Existing automated methods face challenges such as low feature expression capability, poor interpretability, and redundant feature extraction when processing high-dimensional microimage data. We propose a novel fine-grained classification model, SCKansformer, for bone marrow blood cells, which addresses these challenges and enhances classification accuracy and efficiency. The model integrates the Kansformer Encoder, SCConv Encoder, and Global-Local Attention Encoder. The Kansformer Encoder replaces the traditional MLP layer with the KAN, improving nonlinear feature representation and interpretability. The SCConv Encoder, with its Spatial and Channel Reconstruction Units, enhances feature representation and reduces redundancy. The Global-Local Attention Encoder combines Multi-head Self-Attention with a Local Part module to capture both global and local features. We validated our model using the Bone Marrow Blood Cell Fine-Grained Classification Dataset (BMCD-FGCD), comprising over 10,000 samples and nearly 40 classifications, developed with a partner hospital. Comparative experiments on our private dataset, as well as the publicly available PBC and ALL-IDB datasets, demonstrate that SCKansformer outperforms both typical and advanced microcell classification methods across all datasets. Our source code and private BMCD-FGCD dataset are available at https://github.com/JustlfC03/SCKansformer.
CEKD:Cross Ensemble Knowledge Distillation for Augmented Fine-grained Data
Mar 13, 2022
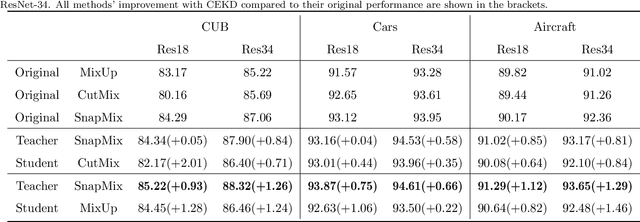
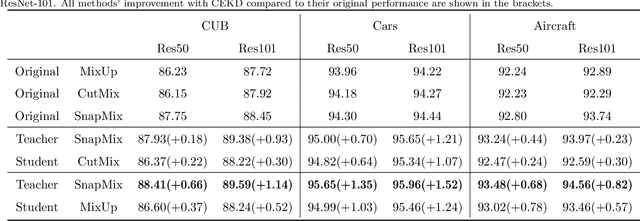

Abstract:Data augmentation has been proved effective in training deep models. Existing data augmentation methods tackle the fine-grained problem by blending image pairs and fusing corresponding labels according to the statistics of mixed pixels, which produces additional noise harmful to the performance of networks. Motivated by this, we present a simple yet effective cross ensemble knowledge distillation (CEKD) model for fine-grained feature learning. We innovatively propose a cross distillation module to provide additional supervision to alleviate the noise problem, and propose a collaborative ensemble module to overcome the target conflict problem. The proposed model can be trained in an end-to-end manner, and only requires image-level label supervision. Extensive experiments on widely used fine-grained benchmarks demonstrate the effectiveness of our proposed model. Specifically, with the backbone of ResNet-101, CEKD obtains the accuracy of 89.59%, 95.96% and 94.56% in three datasets respectively, outperforming state-of-the-art API-Net by 0.99%, 1.06% and 1.16%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge