Avinatan Hassidim
Google Research, Tel-Aviv, Israel
WAXAL: A Large-Scale Multilingual African Language Speech Corpus
Feb 02, 2026Abstract:The advancement of speech technology has predominantly favored high-resource languages, creating a significant digital divide for speakers of most Sub-Saharan African languages. To address this gap, we introduce WAXAL, a large-scale, openly accessible speech dataset for 21 languages representing over 100 million speakers. The collection consists of two main components: an Automated Speech Recognition (ASR) dataset containing approximately 1,250 hours of transcribed, natural speech from a diverse range of speakers, and a Text-to-Speech (TTS) dataset with over 180 hours of high-quality, single-speaker recordings reading phonetically balanced scripts. This paper details our methodology for data collection, annotation, and quality control, which involved partnerships with four African academic and community organizations. We provide a detailed statistical overview of the dataset and discuss its potential limitations and ethical considerations. The WAXAL datasets are released at https://huggingface.co/datasets/google/WaxalNLP under the permissive CC-BY-4.0 license to catalyze research, enable the development of inclusive technologies, and serve as a vital resource for the digital preservation of these languages.
The FACTS Leaderboard: A Comprehensive Benchmark for Large Language Model Factuality
Dec 11, 2025Abstract:We introduce The FACTS Leaderboard, an online leaderboard suite and associated set of benchmarks that comprehensively evaluates the ability of language models to generate factually accurate text across diverse scenarios. The suite provides a holistic measure of factuality by aggregating the performance of models on four distinct sub-leaderboards: (1) FACTS Multimodal, which measures the factuality of responses to image-based questions; (2) FACTS Parametric, which assesses models' world knowledge by answering closed-book factoid questions from internal parameters; (3) FACTS Search, which evaluates factuality in information-seeking scenarios, where the model must use a search API; and (4) FACTS Grounding (v2), which evaluates whether long-form responses are grounded in provided documents, featuring significantly improved judge models. Each sub-leaderboard employs automated judge models to score model responses, and the final suite score is an average of the four components, designed to provide a robust and balanced assessment of a model's overall factuality. The FACTS Leaderboard Suite will be actively maintained, containing both public and private splits to allow for external participation while guarding its integrity. It can be found at https://www.kaggle.com/benchmarks/google/facts .
Advancing Conversational Diagnostic AI with Multimodal Reasoning
May 06, 2025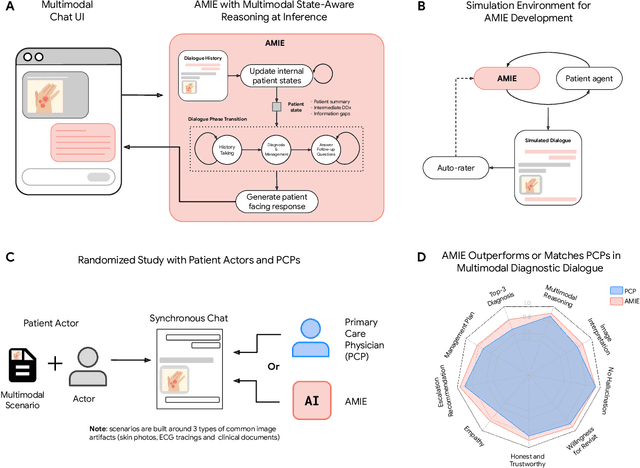
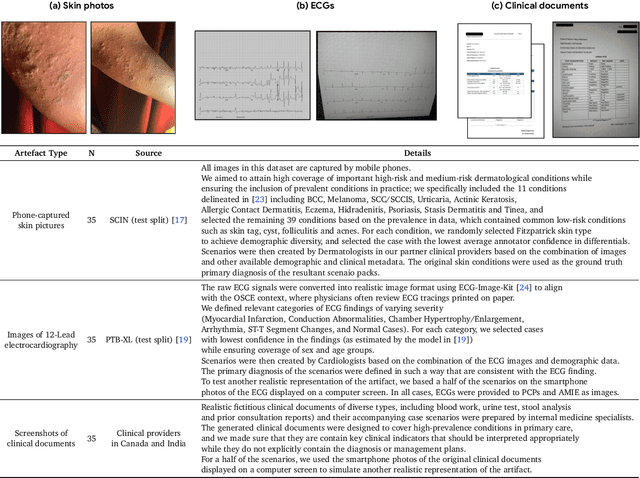
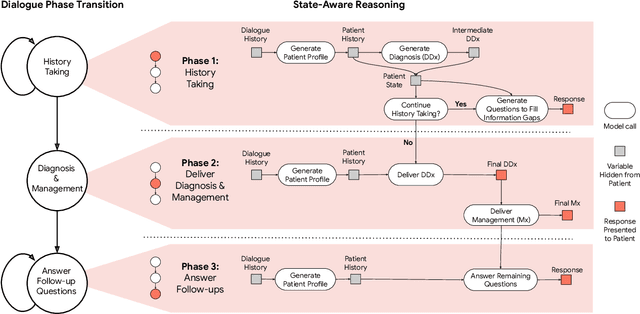
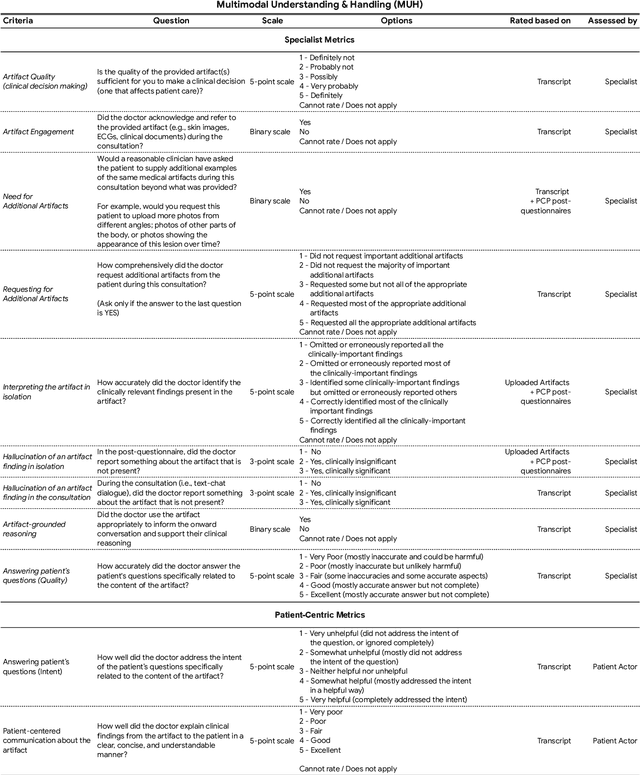
Abstract:Large Language Models (LLMs) have demonstrated great potential for conducting diagnostic conversations but evaluation has been largely limited to language-only interactions, deviating from the real-world requirements of remote care delivery. Instant messaging platforms permit clinicians and patients to upload and discuss multimodal medical artifacts seamlessly in medical consultation, but the ability of LLMs to reason over such data while preserving other attributes of competent diagnostic conversation remains unknown. Here we advance the conversational diagnosis and management performance of the Articulate Medical Intelligence Explorer (AMIE) through a new capability to gather and interpret multimodal data, and reason about this precisely during consultations. Leveraging Gemini 2.0 Flash, our system implements a state-aware dialogue framework, where conversation flow is dynamically controlled by intermediate model outputs reflecting patient states and evolving diagnoses. Follow-up questions are strategically directed by uncertainty in such patient states, leading to a more structured multimodal history-taking process that emulates experienced clinicians. We compared AMIE to primary care physicians (PCPs) in a randomized, blinded, OSCE-style study of chat-based consultations with patient actors. We constructed 105 evaluation scenarios using artifacts like smartphone skin photos, ECGs, and PDFs of clinical documents across diverse conditions and demographics. Our rubric assessed multimodal capabilities and other clinically meaningful axes like history-taking, diagnostic accuracy, management reasoning, communication, and empathy. Specialist evaluation showed AMIE to be superior to PCPs on 7/9 multimodal and 29/32 non-multimodal axes (including diagnostic accuracy). The results show clear progress in multimodal conversational diagnostic AI, but real-world translation needs further research.
LLM-based Text Simplification and its Effect on User Comprehension and Cognitive Load
May 04, 2025Abstract:Information on the web, such as scientific publications and Wikipedia, often surpasses users' reading level. To help address this, we used a self-refinement approach to develop a LLM capability for minimally lossy text simplification. To validate our approach, we conducted a randomized study involving 4563 participants and 31 texts spanning 6 broad subject areas: PubMed (biomedical scientific articles), biology, law, finance, literature/philosophy, and aerospace/computer science. Participants were randomized to viewing original or simplified texts in a subject area, and answered multiple-choice questions (MCQs) that tested their comprehension of the text. The participants were also asked to provide qualitative feedback such as task difficulty. Our results indicate that participants who read the simplified text answered more MCQs correctly than their counterparts who read the original text (3.9% absolute increase, p<0.05). This gain was most striking with PubMed (14.6%), while more moderate gains were observed for finance (5.5%), aerospace/computer science (3.8%) domains, and legal (3.5%). Notably, the results were robust to whether participants could refer back to the text while answering MCQs. The absolute accuracy decreased by up to ~9% for both original and simplified setups where participants could not refer back to the text, but the ~4% overall improvement persisted. Finally, participants' self-reported perceived ease based on a simplified NASA Task Load Index was greater for those who read the simplified text (absolute change on a 5-point scale 0.33, p<0.05). This randomized study, involving an order of magnitude more participants than prior works, demonstrates the potential of LLMs to make complex information easier to understand. Our work aims to enable a broader audience to better learn and make use of expert knowledge available on the web, improving information accessibility.
Towards Conversational AI for Disease Management
Mar 08, 2025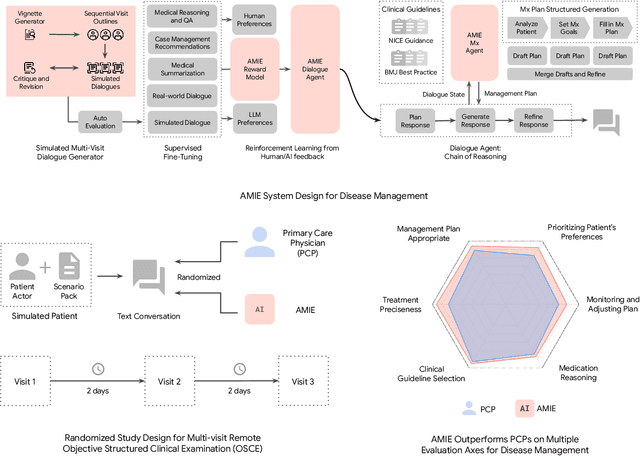

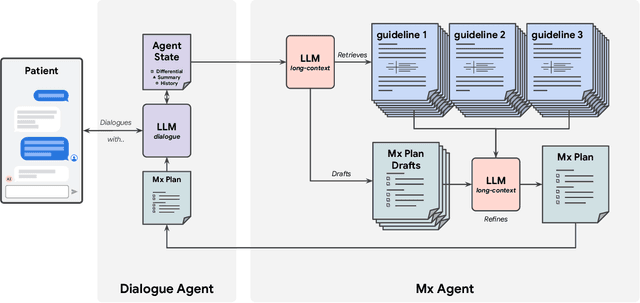
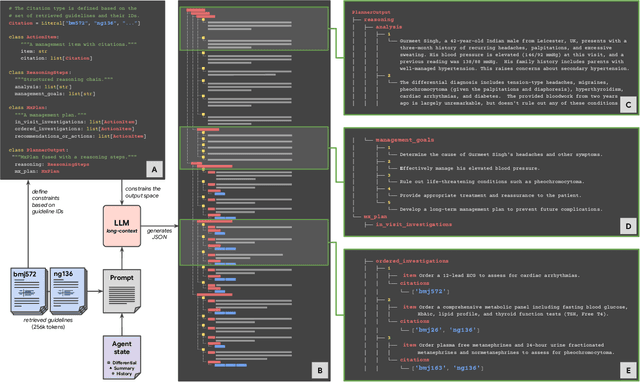
Abstract:While large language models (LLMs) have shown promise in diagnostic dialogue, their capabilities for effective management reasoning - including disease progression, therapeutic response, and safe medication prescription - remain under-explored. We advance the previously demonstrated diagnostic capabilities of the Articulate Medical Intelligence Explorer (AMIE) through a new LLM-based agentic system optimised for clinical management and dialogue, incorporating reasoning over the evolution of disease and multiple patient visit encounters, response to therapy, and professional competence in medication prescription. To ground its reasoning in authoritative clinical knowledge, AMIE leverages Gemini's long-context capabilities, combining in-context retrieval with structured reasoning to align its output with relevant and up-to-date clinical practice guidelines and drug formularies. In a randomized, blinded virtual Objective Structured Clinical Examination (OSCE) study, AMIE was compared to 21 primary care physicians (PCPs) across 100 multi-visit case scenarios designed to reflect UK NICE Guidance and BMJ Best Practice guidelines. AMIE was non-inferior to PCPs in management reasoning as assessed by specialist physicians and scored better in both preciseness of treatments and investigations, and in its alignment with and grounding of management plans in clinical guidelines. To benchmark medication reasoning, we developed RxQA, a multiple-choice question benchmark derived from two national drug formularies (US, UK) and validated by board-certified pharmacists. While AMIE and PCPs both benefited from the ability to access external drug information, AMIE outperformed PCPs on higher difficulty questions. While further research would be needed before real-world translation, AMIE's strong performance across evaluations marks a significant step towards conversational AI as a tool in disease management.
ECLeKTic: a Novel Challenge Set for Evaluation of Cross-Lingual Knowledge Transfer
Feb 28, 2025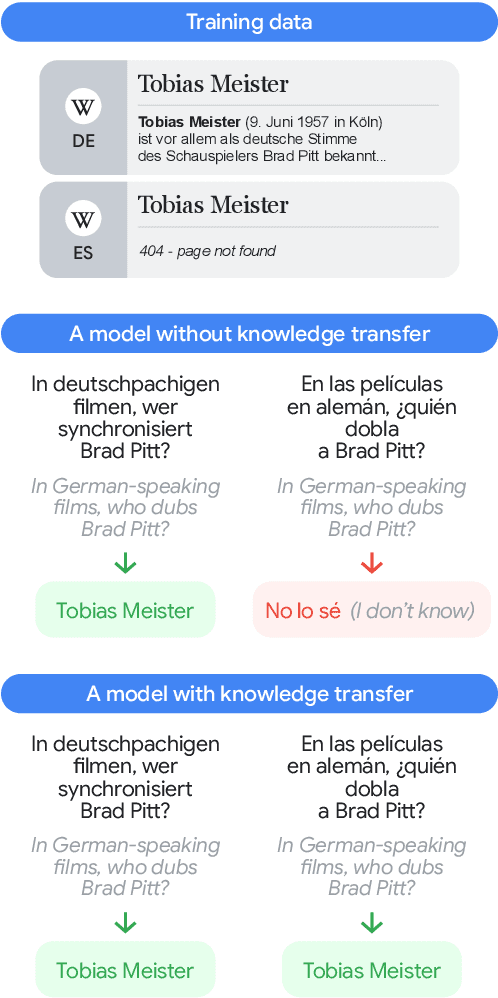
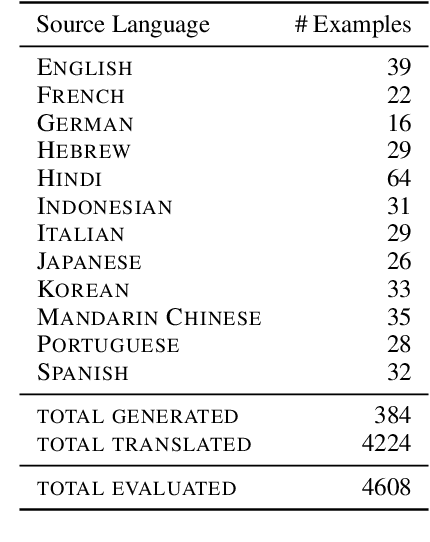
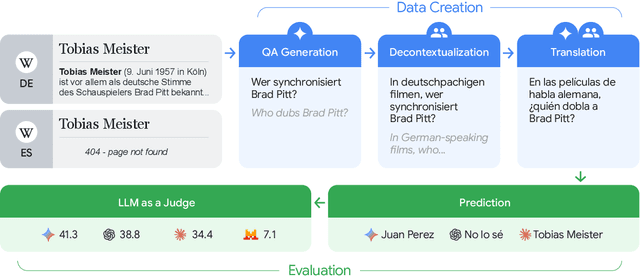
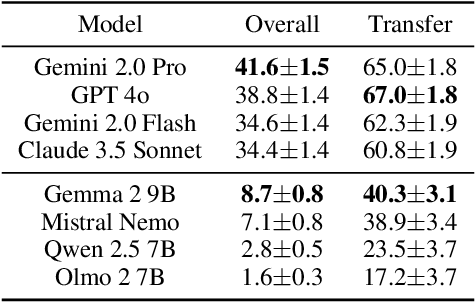
Abstract:To achieve equitable performance across languages, multilingual large language models (LLMs) must be able to abstract knowledge beyond the language in which it was acquired. However, the current literature lacks reliable ways to measure LLMs' capability of cross-lingual knowledge transfer. To that end, we present ECLeKTic, a multilingual closed-book QA (CBQA) dataset that Evaluates Cross-Lingual Knowledge Transfer in a simple, black-box manner. We detected information with uneven coverage across languages by controlling for presence and absence of Wikipedia articles in 12 languages. We generated knowledge-seeking questions in a source language, for which the answer appears in a relevant Wikipedia article and translated them to all other 11 languages, for which the respective Wikipedias lack equivalent articles. Assuming that Wikipedia reflects the prominent knowledge in the LLM's training data, to solve ECLeKTic's CBQA task the model is required to transfer knowledge between languages. Experimenting with 8 LLMs, we show that SOTA models struggle to effectively share knowledge across, languages even if they can predict the answer well for queries in the same language the knowledge was acquired in.
CoCa-CXR: Contrastive Captioners Learn Strong Temporal Structures for Chest X-Ray Vision-Language Understanding
Feb 27, 2025Abstract:Vision-language models have proven to be of great benefit for medical image analysis since they learn rich semantics from both images and reports. Prior efforts have focused on better alignment of image and text representations to enhance image understanding. However, though explicit reference to a prior image is common in Chest X-Ray (CXR) reports, aligning progression descriptions with the semantics differences in image pairs remains under-explored. In this work, we propose two components to address this issue. (1) A CXR report processing pipeline to extract temporal structure. It processes reports with a large language model (LLM) to separate the description and comparison contexts, and extracts fine-grained annotations from reports. (2) A contrastive captioner model for CXR, namely CoCa-CXR, to learn how to both describe images and their temporal progressions. CoCa-CXR incorporates a novel regional cross-attention module to identify local differences between paired CXR images. Extensive experiments show the superiority of CoCa-CXR on both progression analysis and report generation compared to previous methods. Notably, on MS-CXR-T progression classification, CoCa-CXR obtains 65.0% average testing accuracy on five pulmonary conditions, outperforming the previous state-of-the-art (SOTA) model BioViL-T by 4.8%. It also achieves a RadGraph F1 of 24.2% on MIMIC-CXR, which is comparable to the Med-Gemini foundation model.
Towards an AI co-scientist
Feb 26, 2025Abstract:Scientific discovery relies on scientists generating novel hypotheses that undergo rigorous experimental validation. To augment this process, we introduce an AI co-scientist, a multi-agent system built on Gemini 2.0. The AI co-scientist is intended to help uncover new, original knowledge and to formulate demonstrably novel research hypotheses and proposals, building upon prior evidence and aligned to scientist-provided research objectives and guidance. The system's design incorporates a generate, debate, and evolve approach to hypothesis generation, inspired by the scientific method and accelerated by scaling test-time compute. Key contributions include: (1) a multi-agent architecture with an asynchronous task execution framework for flexible compute scaling; (2) a tournament evolution process for self-improving hypotheses generation. Automated evaluations show continued benefits of test-time compute, improving hypothesis quality. While general purpose, we focus development and validation in three biomedical areas: drug repurposing, novel target discovery, and explaining mechanisms of bacterial evolution and anti-microbial resistance. For drug repurposing, the system proposes candidates with promising validation findings, including candidates for acute myeloid leukemia that show tumor inhibition in vitro at clinically applicable concentrations. For novel target discovery, the AI co-scientist proposed new epigenetic targets for liver fibrosis, validated by anti-fibrotic activity and liver cell regeneration in human hepatic organoids. Finally, the AI co-scientist recapitulated unpublished experimental results via a parallel in silico discovery of a novel gene transfer mechanism in bacterial evolution. These results, detailed in separate, co-timed reports, demonstrate the potential to augment biomedical and scientific discovery and usher an era of AI empowered scientists.
Health AI Developer Foundations
Nov 26, 2024



Abstract:Robust medical Machine Learning (ML) models have the potential to revolutionize healthcare by accelerating clinical research, improving workflows and outcomes, and producing novel insights or capabilities. Developing such ML models from scratch is cost prohibitive and requires substantial compute, data, and time (e.g., expert labeling). To address these challenges, we introduce Health AI Developer Foundations (HAI-DEF), a suite of pre-trained, domain-specific foundation models, tools, and recipes to accelerate building ML for health applications. The models cover various modalities and domains, including radiology (X-rays and computed tomography), histopathology, dermatological imaging, and audio. These models provide domain specific embeddings that facilitate AI development with less labeled data, shorter training times, and reduced computational costs compared to traditional approaches. In addition, we utilize a common interface and style across these models, and prioritize usability to enable developers to integrate HAI-DEF efficiently. We present model evaluations across various tasks and conclude with a discussion of their application and evaluation, covering the importance of ensuring efficacy, fairness, and equity. Finally, while HAI-DEF and specifically the foundation models lower the barrier to entry for ML in healthcare, we emphasize the importance of validation with problem- and population-specific data for each desired usage setting. This technical report will be updated over time as more modalities and features are added.
Can Few-shot Work in Long-Context? Recycling the Context to Generate Demonstrations
Jun 19, 2024Abstract:Despite recent advancements in Large Language Models (LLMs), their performance on tasks involving long contexts remains sub-optimal. In-Context Learning (ICL) with few-shot examples may be an appealing solution to enhance LLM performance in this scenario; However, naively adding ICL examples with long context introduces challenges, including substantial token overhead added for each few-shot example and context mismatch between the demonstrations and the target query. In this work, we propose to automatically generate few-shot examples for long context QA tasks by recycling contexts. Specifically, given a long input context (1-3k tokens) and a query, we generate additional query-output pairs from the given context as few-shot examples, while introducing the context only once. This ensures that the demonstrations are leveraging the same context as the target query while only adding a small number of tokens to the prompt. We further enhance each demonstration by instructing the model to explicitly identify the relevant paragraphs before the answer, which improves performance while providing fine-grained attribution to the answer source. We apply our method on multiple LLMs and obtain substantial improvements on various QA datasets with long context, especially when the answer lies within the middle of the context. Surprisingly, despite introducing only single-hop ICL examples, LLMs also successfully generalize to multi-hop long-context QA using our approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge