Rickmer Braren
Department of Diagnostic and Interventional Radiology, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Germany
Parametric shape models for vessels learned from segmentations via differentiable voxelization
Jul 03, 2025Abstract:Vessels are complex structures in the body that have been studied extensively in multiple representations. While voxelization is the most common of them, meshes and parametric models are critical in various applications due to their desirable properties. However, these representations are typically extracted through segmentations and used disjointly from each other. We propose a framework that joins the three representations under differentiable transformations. By leveraging differentiable voxelization, we automatically extract a parametric shape model of the vessels through shape-to-segmentation fitting, where we learn shape parameters from segmentations without the explicit need for ground-truth shape parameters. The vessel is parametrized as centerlines and radii using cubic B-splines, ensuring smoothness and continuity by construction. Meshes are differentiably extracted from the learned shape parameters, resulting in high-fidelity meshes that can be manipulated post-fit. Our method can accurately capture the geometry of complex vessels, as demonstrated by the volumetric fits in experiments on aortas, aneurysms, and brain vessels.
Automated Thoracolumbar Stump Rib Detection and Analysis in a Large CT Cohort
May 08, 2025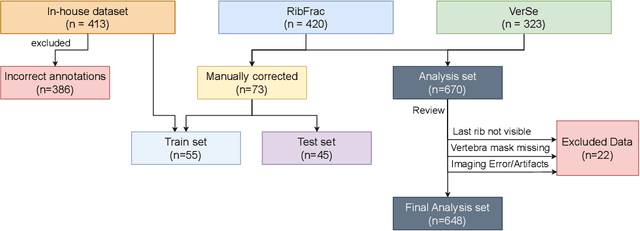
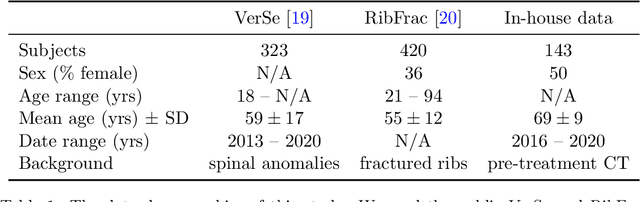
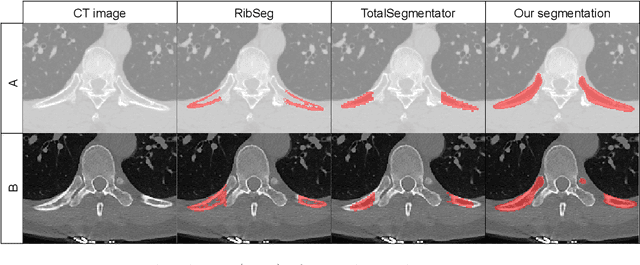
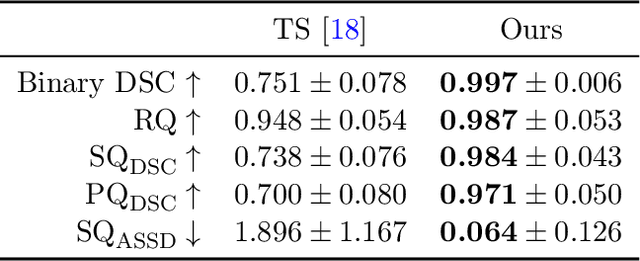
Abstract:Thoracolumbar stump ribs are one of the essential indicators of thoracolumbar transitional vertebrae or enumeration anomalies. While some studies manually assess these anomalies and describe the ribs qualitatively, this study aims to automate thoracolumbar stump rib detection and analyze their morphology quantitatively. To this end, we train a high-resolution deep-learning model for rib segmentation and show significant improvements compared to existing models (Dice score 0.997 vs. 0.779, p-value < 0.01). In addition, we use an iterative algorithm and piece-wise linear interpolation to assess the length of the ribs, showing a success rate of 98.2%. When analyzing morphological features, we show that stump ribs articulate more posteriorly at the vertebrae (-19.2 +- 3.8 vs -13.8 +- 2.5, p-value < 0.01), are thinner (260.6 +- 103.4 vs. 563.6 +- 127.1, p-value < 0.01), and are oriented more downwards and sideways within the first centimeters in contrast to full-length ribs. We show that with partially visible ribs, these features can achieve an F1-score of 0.84 in differentiating stump ribs from regular ones. We publish the model weights and masks for public use.
Enhanced Diagnostic Fidelity in Pathology Whole Slide Image Compression via Deep Learning
Mar 14, 2025Abstract:Accurate diagnosis of disease often depends on the exhaustive examination of Whole Slide Images (WSI) at microscopic resolution. Efficient handling of these data-intensive images requires lossy compression techniques. This paper investigates the limitations of the widely-used JPEG algorithm, the current clinical standard, and reveals severe image artifacts impacting diagnostic fidelity. To overcome these challenges, we introduce a novel deep-learning (DL)-based compression method tailored for pathology images. By enforcing feature similarity of deep features between the original and compressed images, our approach achieves superior Peak Signal-to-Noise Ratio (PSNR), Multi-Scale Structural Similarity Index (MS-SSIM), and Learned Perceptual Image Patch Similarity (LPIPS) scores compared to JPEG-XL, Webp, and other DL compression methods.
Self-Supervised Radiograph Anatomical Region Classification -- How Clean Is Your Real-World Data?
Dec 20, 2024Abstract:Modern deep learning-based clinical imaging workflows rely on accurate labels of the examined anatomical region. Knowing the anatomical region is required to select applicable downstream models and to effectively generate cohorts of high quality data for future medical and machine learning research efforts. However, this information may not be available in externally sourced data or generally contain data entry errors. To address this problem, we show the effectiveness of self-supervised methods such as SimCLR and BYOL as well as supervised contrastive deep learning methods in assigning one of 14 anatomical region classes in our in-house dataset of 48,434 skeletal radiographs. We achieve a strong linear evaluation accuracy of 96.6% with a single model and 97.7% using an ensemble approach. Furthermore, only a few labeled instances (1% of the training set) suffice to achieve an accuracy of 92.2%, enabling usage in low-label and thus low-resource scenarios. Our model can be used to correct data entry mistakes: a follow-up analysis of the test set errors of our best-performing single model by an expert radiologist identified 35% incorrect labels and 11% out-of-domain images. When accounted for, the radiograph anatomical region labelling performance increased -- without and with an ensemble, respectively -- to a theoretical accuracy of 98.0% and 98.8%.
Unlocking the Potential of Digital Pathology: Novel Baselines for Compression
Dec 17, 2024



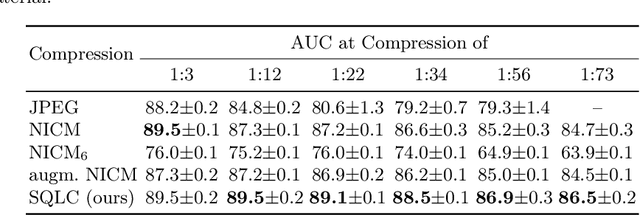
Abstract:Digital pathology offers a groundbreaking opportunity to transform clinical practice in histopathological image analysis, yet faces a significant hurdle: the substantial file sizes of pathological Whole Slide Images (WSI). While current digital pathology solutions rely on lossy JPEG compression to address this issue, lossy compression can introduce color and texture disparities, potentially impacting clinical decision-making. While prior research addresses perceptual image quality and downstream performance independently of each other, we jointly evaluate compression schemes for perceptual and downstream task quality on four different datasets. In addition, we collect an initially uncompressed dataset for an unbiased perceptual evaluation of compression schemes. Our results show that deep learning models fine-tuned for perceptual quality outperform conventional compression schemes like JPEG-XL or WebP for further compression of WSI. However, they exhibit a significant bias towards the compression artifacts present in the training data and struggle to generalize across various compression schemes. We introduce a novel evaluation metric based on feature similarity between original files and compressed files that aligns very well with the actual downstream performance on the compressed WSI. Our metric allows for a general and standardized evaluation of lossy compression schemes and mitigates the requirement to independently assess different downstream tasks. Our study provides novel insights for the assessment of lossy compression schemes for WSI and encourages a unified evaluation of lossy compression schemes to accelerate the clinical uptake of digital pathology.
General Vision Encoder Features as Guidance in Medical Image Registration
Jul 18, 2024Abstract:General vision encoders like DINOv2 and SAM have recently transformed computer vision. Even though they are trained on natural images, such encoder models have excelled in medical imaging, e.g., in classification, segmentation, and registration. However, no in-depth comparison of different state-of-the-art general vision encoders for medical registration is available. In this work, we investigate how well general vision encoder features can be used in the dissimilarity metrics for medical image registration. We explore two encoders that were trained on natural images as well as one that was fine-tuned on medical data. We apply the features within the well-established B-spline FFD registration framework. In extensive experiments on cardiac cine MRI data, we find that using features as additional guidance for conventional metrics improves the registration quality. The code is available at github.com/compai-lab/2024-miccai-koegl.
Data-Driven Tissue- and Subject-Specific Elastic Regularization for Medical Image Registration
Jul 05, 2024
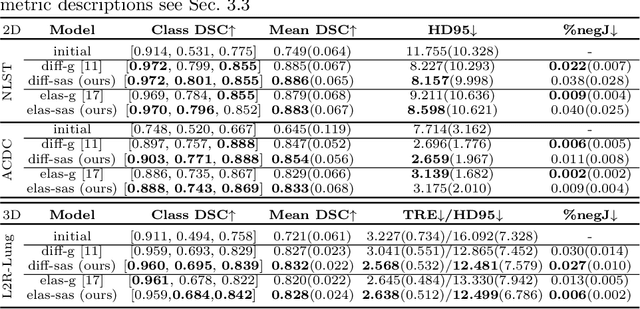
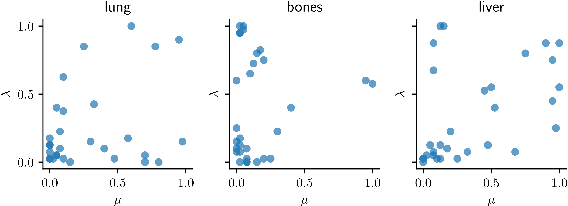
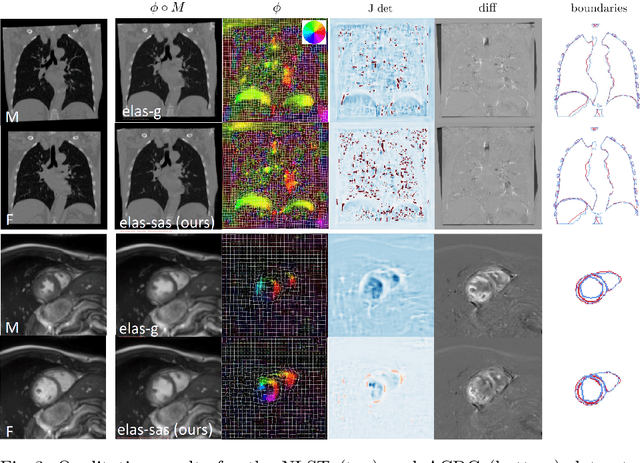
Abstract:Physics-inspired regularization is desired for intra-patient image registration since it can effectively capture the biomechanical characteristics of anatomical structures. However, a major challenge lies in the reliance on physical parameters: Parameter estimations vary widely across the literature, and the physical properties themselves are inherently subject-specific. In this work, we introduce a novel data-driven method that leverages hypernetworks to learn the tissue-dependent elasticity parameters of an elastic regularizer. Notably, our approach facilitates the estimation of patient-specific parameters without the need to retrain the network. We evaluate our method on three publicly available 2D and 3D lung CT and cardiac MR datasets. We find that with our proposed subject-specific tissue-dependent regularization, a higher registration quality is achieved across all datasets compared to using a global regularizer. The code is available at https://github.com/compai-lab/2024-miccai-reithmeir.
Learned Image Compression for HE-stained Histopathological Images via Stain Deconvolution
Jun 18, 2024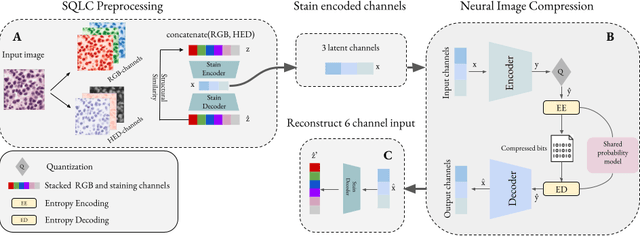
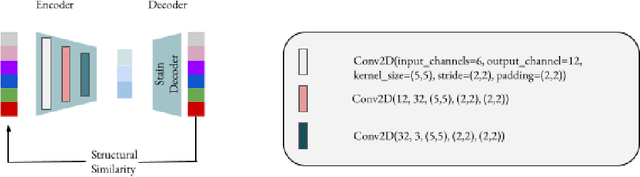
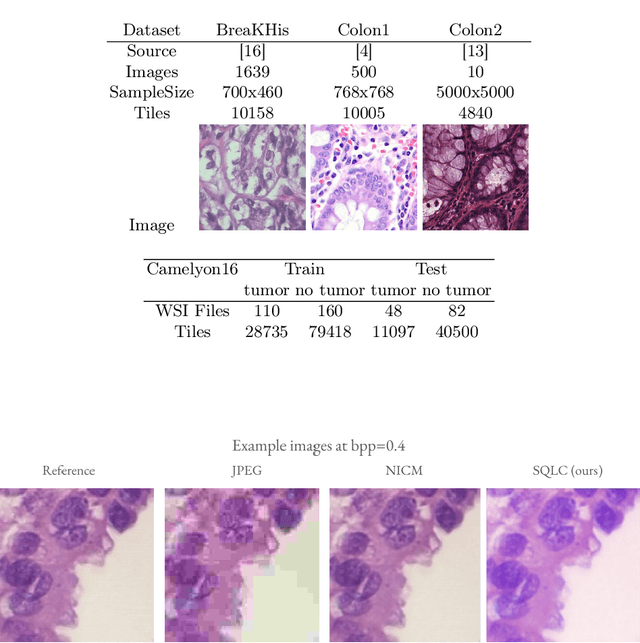
Abstract:Processing histopathological Whole Slide Images (WSI) leads to massive storage requirements for clinics worldwide. Even after lossy image compression during image acquisition, additional lossy compression is frequently possible without substantially affecting the performance of deep learning-based (DL) downstream tasks. In this paper, we show that the commonly used JPEG algorithm is not best suited for further compression and we propose Stain Quantized Latent Compression (SQLC ), a novel DL based histopathology data compression approach. SQLC compresses staining and RGB channels before passing it through a compression autoencoder (CAE ) in order to obtain quantized latent representations for maximizing the compression. We show that our approach yields superior performance in a classification downstream task, compared to traditional approaches like JPEG, while image quality metrics like the Multi-Scale Structural Similarity Index (MS-SSIM) is largely preserved. Our method is online available.
Real-World Federated Learning in Radiology: Hurdles to overcome and Benefits to gain
May 15, 2024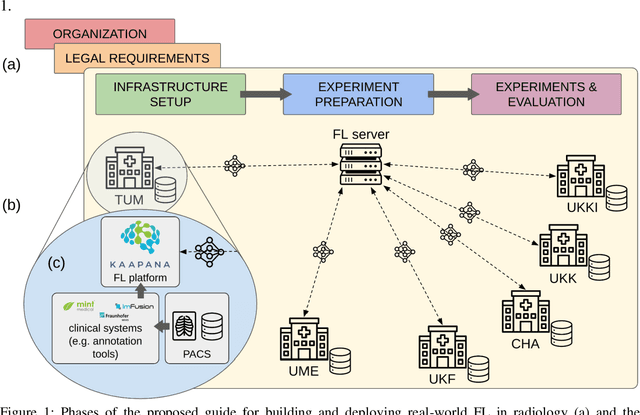



Abstract:Objective: Federated Learning (FL) enables collaborative model training while keeping data locally. Currently, most FL studies in radiology are conducted in simulated environments due to numerous hurdles impeding its translation into practice. The few existing real-world FL initiatives rarely communicate specific measures taken to overcome these hurdles, leaving behind a significant knowledge gap. Minding efforts to implement real-world FL, there is a notable lack of comprehensive assessment comparing FL to less complex alternatives. Materials & Methods: We extensively reviewed FL literature, categorizing insights along with our findings according to their nature and phase while establishing a FL initiative, summarized to a comprehensive guide. We developed our own FL infrastructure within the German Radiological Cooperative Network (RACOON) and demonstrated its functionality by training FL models on lung pathology segmentation tasks across six university hospitals. We extensively evaluated FL against less complex alternatives in three distinct evaluation scenarios. Results: The proposed guide outlines essential steps, identified hurdles, and proposed solutions for establishing successful FL initiatives conducting real-world experiments. Our experimental results show that FL outperforms less complex alternatives in all evaluation scenarios, justifying the effort required to translate FL into real-world applications. Discussion & Conclusion: Our proposed guide aims to aid future FL researchers in circumventing pitfalls and accelerating translation of FL into radiological applications. Our results underscore the value of efforts needed to translate FL into real-world applications by demonstrating advantageous performance over alternatives, and emphasize the importance of strategic organization, robust management of distributed data and infrastructure in real-world settings.
Reconciling AI Performance and Data Reconstruction Resilience for Medical Imaging
Dec 05, 2023



Abstract:Artificial Intelligence (AI) models are vulnerable to information leakage of their training data, which can be highly sensitive, for example in medical imaging. Privacy Enhancing Technologies (PETs), such as Differential Privacy (DP), aim to circumvent these susceptibilities. DP is the strongest possible protection for training models while bounding the risks of inferring the inclusion of training samples or reconstructing the original data. DP achieves this by setting a quantifiable privacy budget. Although a lower budget decreases the risk of information leakage, it typically also reduces the performance of such models. This imposes a trade-off between robust performance and stringent privacy. Additionally, the interpretation of a privacy budget remains abstract and challenging to contextualize. In this study, we contrast the performance of AI models at various privacy budgets against both, theoretical risk bounds and empirical success of reconstruction attacks. We show that using very large privacy budgets can render reconstruction attacks impossible, while drops in performance are negligible. We thus conclude that not using DP -- at all -- is negligent when applying AI models to sensitive data. We deem those results to lie a foundation for further debates on striking a balance between privacy risks and model performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge