Ünal Akünal
Division of Medical Image Computing, German Cancer Research Center, Heidelberg, Germany
Kaapana: A Comprehensive Open-Source Platform for Integrating AI in Medical Imaging Research Environments
Dec 10, 2025
Abstract:Developing generalizable AI for medical imaging requires both access to large, multi-center datasets and standardized, reproducible tooling within research environments. However, leveraging real-world imaging data in clinical research environments is still hampered by strict regulatory constraints, fragmented software infrastructure, and the challenges inherent in conducting large-cohort multicentre studies. This leads to projects that rely on ad-hoc toolchains that are hard to reproduce, difficult to scale beyond single institutions and poorly suited for collaboration between clinicians and data scientists. We present Kaapana, a comprehensive open-source platform for medical imaging research that is designed to bridge this gap. Rather than building single-use, site-specific tooling, Kaapana provides a modular, extensible framework that unifies data ingestion, cohort curation, processing workflows and result inspection under a common user interface. By bringing the algorithm to the data, it enables institutions to keep control over their sensitive data while still participating in distributed experimentation and model development. By integrating flexible workflow orchestration with user-facing applications for researchers, Kaapana reduces technical overhead, improves reproducibility and enables conducting large-scale, collaborative, multi-centre imaging studies. We describe the core concepts of the platform and illustrate how they can support diverse use cases, from local prototyping to nation-wide research networks. The open-source codebase is available at https://github.com/kaapana/kaapana
Fair Evaluation of Federated Learning Algorithms for Automated Breast Density Classification: The Results of the 2022 ACR-NCI-NVIDIA Federated Learning Challenge
May 22, 2024Abstract:The correct interpretation of breast density is important in the assessment of breast cancer risk. AI has been shown capable of accurately predicting breast density, however, due to the differences in imaging characteristics across mammography systems, models built using data from one system do not generalize well to other systems. Though federated learning (FL) has emerged as a way to improve the generalizability of AI without the need to share data, the best way to preserve features from all training data during FL is an active area of research. To explore FL methodology, the breast density classification FL challenge was hosted in partnership with the American College of Radiology, Harvard Medical School's Mass General Brigham, University of Colorado, NVIDIA, and the National Institutes of Health National Cancer Institute. Challenge participants were able to submit docker containers capable of implementing FL on three simulated medical facilities, each containing a unique large mammography dataset. The breast density FL challenge ran from June 15 to September 5, 2022, attracting seven finalists from around the world. The winning FL submission reached a linear kappa score of 0.653 on the challenge test data and 0.413 on an external testing dataset, scoring comparably to a model trained on the same data in a central location.
* 16 pages, 9 figures
Real-World Federated Learning in Radiology: Hurdles to overcome and Benefits to gain
May 15, 2024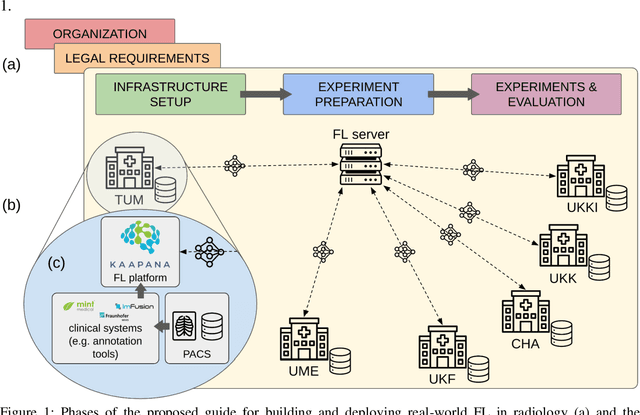



Abstract:Objective: Federated Learning (FL) enables collaborative model training while keeping data locally. Currently, most FL studies in radiology are conducted in simulated environments due to numerous hurdles impeding its translation into practice. The few existing real-world FL initiatives rarely communicate specific measures taken to overcome these hurdles, leaving behind a significant knowledge gap. Minding efforts to implement real-world FL, there is a notable lack of comprehensive assessment comparing FL to less complex alternatives. Materials & Methods: We extensively reviewed FL literature, categorizing insights along with our findings according to their nature and phase while establishing a FL initiative, summarized to a comprehensive guide. We developed our own FL infrastructure within the German Radiological Cooperative Network (RACOON) and demonstrated its functionality by training FL models on lung pathology segmentation tasks across six university hospitals. We extensively evaluated FL against less complex alternatives in three distinct evaluation scenarios. Results: The proposed guide outlines essential steps, identified hurdles, and proposed solutions for establishing successful FL initiatives conducting real-world experiments. Our experimental results show that FL outperforms less complex alternatives in all evaluation scenarios, justifying the effort required to translate FL into real-world applications. Discussion & Conclusion: Our proposed guide aims to aid future FL researchers in circumventing pitfalls and accelerating translation of FL into radiological applications. Our results underscore the value of efforts needed to translate FL into real-world applications by demonstrating advantageous performance over alternatives, and emphasize the importance of strategic organization, robust management of distributed data and infrastructure in real-world settings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge