Manuel Nickel
FedPID: An Aggregation Method for Federated Learning
Nov 04, 2024Abstract:This paper presents FedPID, our submission to the Federated Tumor Segmentation Challenge 2024 (FETS24). Inspired by FedCostWAvg and FedPIDAvg, our winning contributions to FETS21 and FETS2022, we propose an improved aggregation strategy for federated and collaborative learning. FedCostWAvg is a method that averages results by considering both the number of training samples in each group and how much the cost function decreased in the last round of training. This is similar to how the derivative part of a PID controller works. In FedPIDAvg, we also included the integral part that was missing. Another challenge we faced were vastly differing dataset sizes at each center. We solved this by assuming the sizes follow a Poisson distribution and adjusting the training iterations for each center accordingly. Essentially, this part of the method controls that outliers that require too much training time are less frequently used. Based on these contributions we now adapted FedPIDAvg by changing how the integral part is computed. Instead of integrating the loss function we measure the global drop in cost since the first round.
Real-World Federated Learning in Radiology: Hurdles to overcome and Benefits to gain
May 15, 2024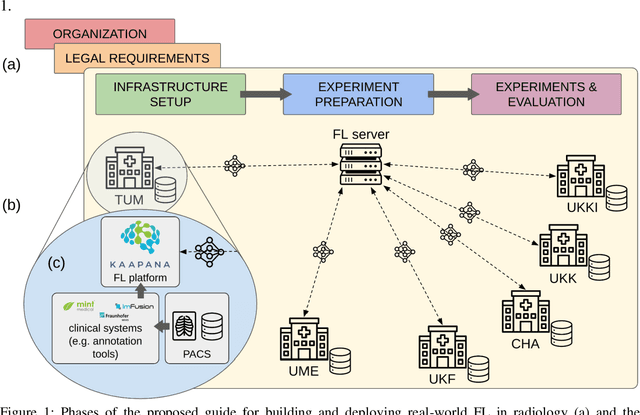



Abstract:Objective: Federated Learning (FL) enables collaborative model training while keeping data locally. Currently, most FL studies in radiology are conducted in simulated environments due to numerous hurdles impeding its translation into practice. The few existing real-world FL initiatives rarely communicate specific measures taken to overcome these hurdles, leaving behind a significant knowledge gap. Minding efforts to implement real-world FL, there is a notable lack of comprehensive assessment comparing FL to less complex alternatives. Materials & Methods: We extensively reviewed FL literature, categorizing insights along with our findings according to their nature and phase while establishing a FL initiative, summarized to a comprehensive guide. We developed our own FL infrastructure within the German Radiological Cooperative Network (RACOON) and demonstrated its functionality by training FL models on lung pathology segmentation tasks across six university hospitals. We extensively evaluated FL against less complex alternatives in three distinct evaluation scenarios. Results: The proposed guide outlines essential steps, identified hurdles, and proposed solutions for establishing successful FL initiatives conducting real-world experiments. Our experimental results show that FL outperforms less complex alternatives in all evaluation scenarios, justifying the effort required to translate FL into real-world applications. Discussion & Conclusion: Our proposed guide aims to aid future FL researchers in circumventing pitfalls and accelerating translation of FL into radiological applications. Our results underscore the value of efforts needed to translate FL into real-world applications by demonstrating advantageous performance over alternatives, and emphasize the importance of strategic organization, robust management of distributed data and infrastructure in real-world settings.
CPS: Class-level 6D Pose and Shape Estimation From Monocular Images
Mar 13, 2020



Abstract:Contemporary monocular 6D pose estimation methods can only cope with a handful of object instances. This naturally limits possible applications as, for instance, robots need to work with hundreds of different objects in a real environment. In this paper, we propose the first deep learning approach for class-wise monocular 6D pose estimation, coupled with metric shape retrieval. We propose a new loss formulation which directly optimizes over all parameters, i.e. 3D orientation, translation, scale and shape at the same time. Instead of decoupling each parameter, we transform the regressed shape, in the form of a point cloud, to 3D and directly measure its metric misalignment. We experimentally demonstrate that we can retrieve precise metric point clouds from a single image, which can also be further processed for e.g. subsequent rendering. Moreover, we show that our new 3D point cloud loss outperforms all baselines and gives overall good results despite the inherent ambiguity due to monocular data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge