Jianfeng Wu
for the Alzheimer's Disease Neuroimaging Initiative
PathFound: An Agentic Multimodal Model Activating Evidence-seeking Pathological Diagnosis
Dec 29, 2025Abstract:Recent pathological foundation models have substantially advanced visual representation learning and multimodal interaction. However, most models still rely on a static inference paradigm in which whole-slide images are processed once to produce predictions, without reassessment or targeted evidence acquisition under ambiguous diagnoses. This contrasts with clinical diagnostic workflows that refine hypotheses through repeated slide observations and further examination requests. We propose PathFound, an agentic multimodal model designed to support evidence-seeking inference in pathological diagnosis. PathFound integrates the power of pathological visual foundation models, vision-language models, and reasoning models trained with reinforcement learning to perform proactive information acquisition and diagnosis refinement by progressing through the initial diagnosis, evidence-seeking, and final decision stages. Across several large multimodal models, adopting this strategy consistently improves diagnostic accuracy, indicating the effectiveness of evidence-seeking workflows in computational pathology. Among these models, PathFound achieves state-of-the-art diagnostic performance across diverse clinical scenarios and demonstrates strong potential to discover subtle details, such as nuclear features and local invasions.
PathBench: A comprehensive comparison benchmark for pathology foundation models towards precision oncology
May 26, 2025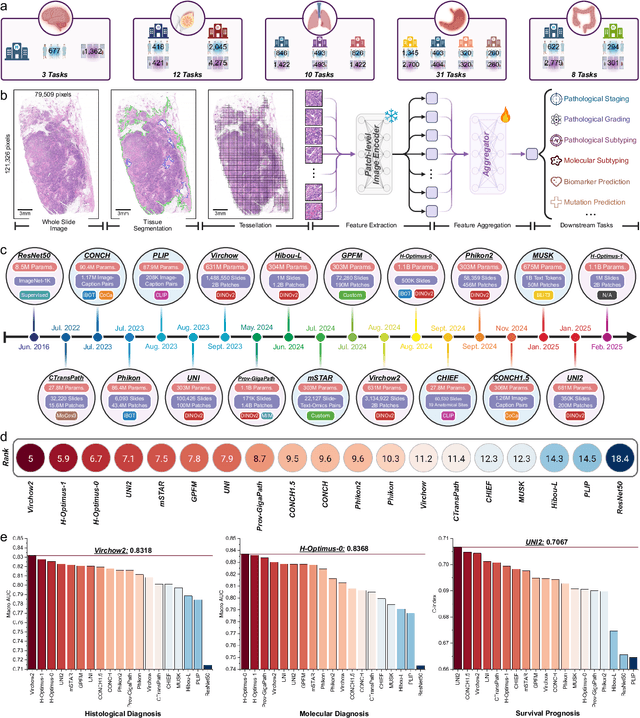



Abstract:The emergence of pathology foundation models has revolutionized computational histopathology, enabling highly accurate, generalized whole-slide image analysis for improved cancer diagnosis, and prognosis assessment. While these models show remarkable potential across cancer diagnostics and prognostics, their clinical translation faces critical challenges including variability in optimal model across cancer types, potential data leakage in evaluation, and lack of standardized benchmarks. Without rigorous, unbiased evaluation, even the most advanced PFMs risk remaining confined to research settings, delaying their life-saving applications. Existing benchmarking efforts remain limited by narrow cancer-type focus, potential pretraining data overlaps, or incomplete task coverage. We present PathBench, the first comprehensive benchmark addressing these gaps through: multi-center in-hourse datasets spanning common cancers with rigorous leakage prevention, evaluation across the full clinical spectrum from diagnosis to prognosis, and an automated leaderboard system for continuous model assessment. Our framework incorporates large-scale data, enabling objective comparison of PFMs while reflecting real-world clinical complexity. All evaluation data comes from private medical providers, with strict exclusion of any pretraining usage to avoid data leakage risks. We have collected 15,888 WSIs from 8,549 patients across 10 hospitals, encompassing over 64 diagnosis and prognosis tasks. Currently, our evaluation of 19 PFMs shows that Virchow2 and H-Optimus-1 are the most effective models overall. This work provides researchers with a robust platform for model development and offers clinicians actionable insights into PFM performance across diverse clinical scenarios, ultimately accelerating the translation of these transformative technologies into routine pathology practice.
PathOrchestra: A Comprehensive Foundation Model for Computational Pathology with Over 100 Diverse Clinical-Grade Tasks
Mar 31, 2025Abstract:The complexity and variability inherent in high-resolution pathological images present significant challenges in computational pathology. While pathology foundation models leveraging AI have catalyzed transformative advancements, their development demands large-scale datasets, considerable storage capacity, and substantial computational resources. Furthermore, ensuring their clinical applicability and generalizability requires rigorous validation across a broad spectrum of clinical tasks. Here, we present PathOrchestra, a versatile pathology foundation model trained via self-supervised learning on a dataset comprising 300K pathological slides from 20 tissue and organ types across multiple centers. The model was rigorously evaluated on 112 clinical tasks using a combination of 61 private and 51 public datasets. These tasks encompass digital slide preprocessing, pan-cancer classification, lesion identification, multi-cancer subtype classification, biomarker assessment, gene expression prediction, and the generation of structured reports. PathOrchestra demonstrated exceptional performance across 27,755 WSIs and 9,415,729 ROIs, achieving over 0.950 accuracy in 47 tasks, including pan-cancer classification across various organs, lymphoma subtype diagnosis, and bladder cancer screening. Notably, it is the first model to generate structured reports for high-incidence colorectal cancer and diagnostically complex lymphoma-areas that are infrequently addressed by foundational models but hold immense clinical potential. Overall, PathOrchestra exemplifies the feasibility and efficacy of a large-scale, self-supervised pathology foundation model, validated across a broad range of clinical-grade tasks. Its high accuracy and reduced reliance on extensive data annotation underline its potential for clinical integration, offering a pathway toward more efficient and high-quality medical services.
Make Full Use of Testing Information: An Integrated Accelerated Testing and Evaluation Method for Autonomous Driving Systems
Jan 21, 2025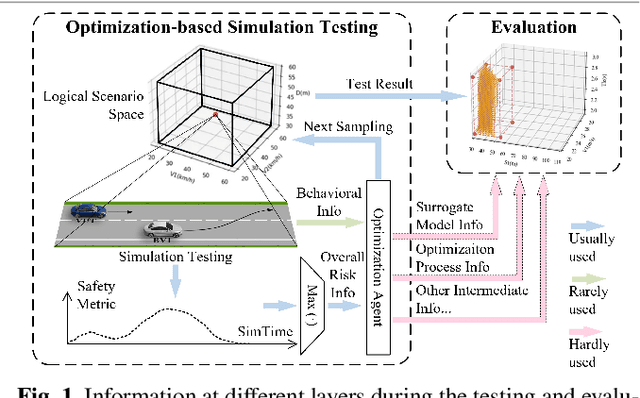
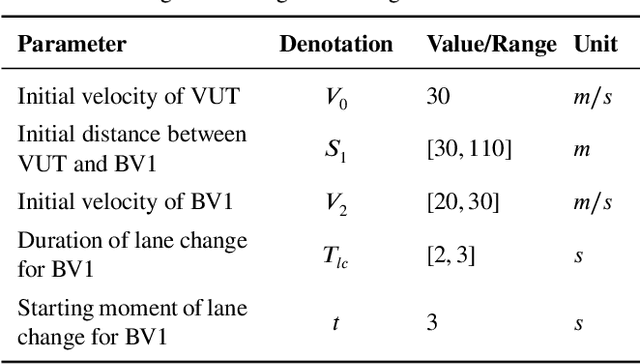
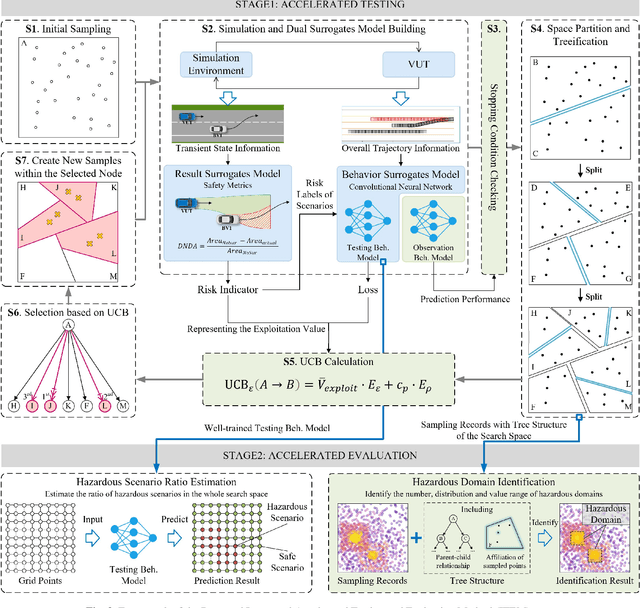
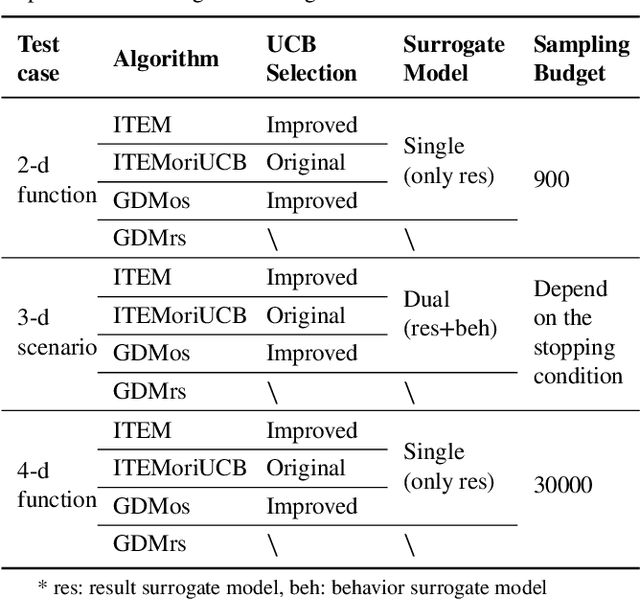
Abstract:Testing and evaluation is an important step before the large-scale application of the autonomous driving systems (ADSs). Based on the three level of scenario abstraction theory, a testing can be performed within a logical scenario, followed by an evaluation stage which is inputted with the testing results of each concrete scenario generated from the logical parameter space. During the above process, abundant testing information is produced which is beneficial for comprehensive and accurate evaluations. To make full use of testing information, this paper proposes an Integrated accelerated Testing and Evaluation Method (ITEM). Based on a Monte Carlo Tree Search (MCTS) paradigm and a dual surrogates testing framework proposed in our previous work, this paper applies the intermediate information (i.e., the tree structure, including the affiliation of each historical sampled point with the subspaces and the parent-child relationship between subspaces) generated during the testing stage into the evaluation stage to achieve accurate hazardous domain identification. Moreover, to better serve this purpose, the UCB calculation method is improved to allow the search algorithm to focus more on the hazardous domain boundaries. Further, a stopping condition is constructed based on the convergence of the search algorithm. Ablation and comparative experiments are then conducted to verify the effectiveness of the improvements and the superiority of the proposed method. The experimental results show that ITEM could well identify the hazardous domains in both low- and high-dimensional cases, regardless of the shape of the hazardous domains, indicating its generality and potential for the safety evaluation of ADSs.
Simple but Effective Compound Geometric Operations for Temporal Knowledge Graph Completion
Aug 13, 2024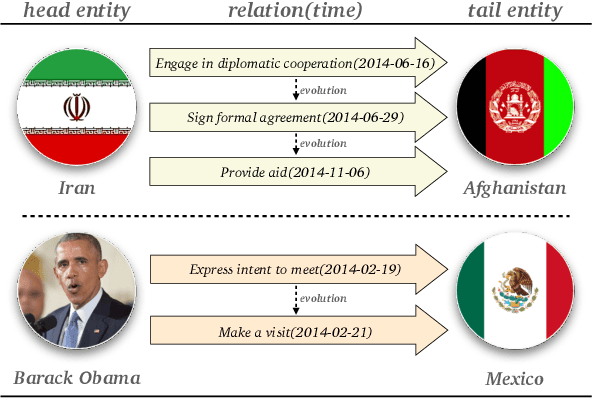
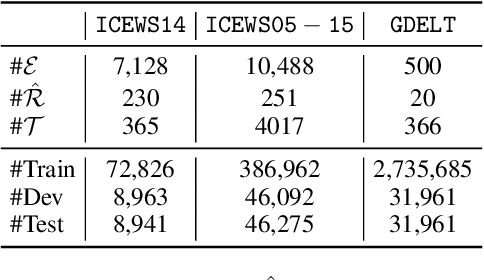
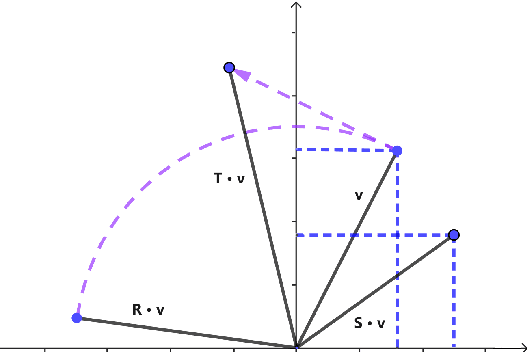
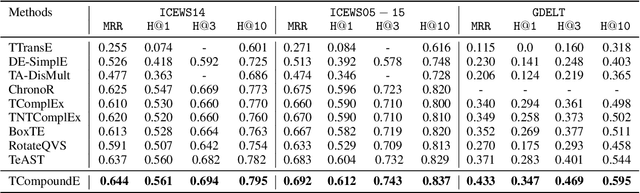
Abstract:Temporal knowledge graph completion aims to infer the missing facts in temporal knowledge graphs. Current approaches usually embed factual knowledge into continuous vector space and apply geometric operations to learn potential patterns in temporal knowledge graphs. However, these methods only adopt a single operation, which may have limitations in capturing the complex temporal dynamics present in temporal knowledge graphs. Therefore, we propose a simple but effective method, i.e. TCompoundE, which is specially designed with two geometric operations, including time-specific and relation-specific operations. We provide mathematical proofs to demonstrate the ability of TCompoundE to encode various relation patterns. Experimental results show that our proposed model significantly outperforms existing temporal knowledge graph embedding models. Our code is available at https://github.com/nk-ruiying/TCompoundE.
Hierarchical-level rain image generative model based on GAN
Sep 06, 2023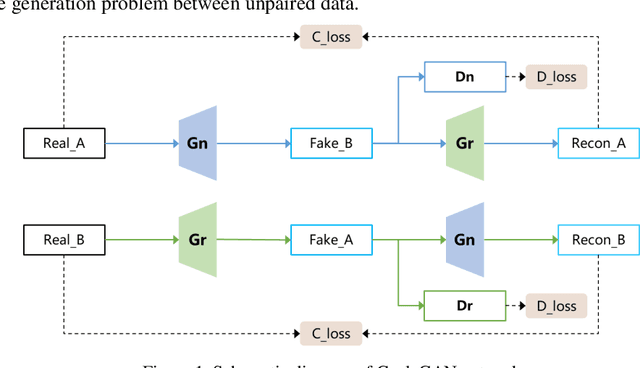
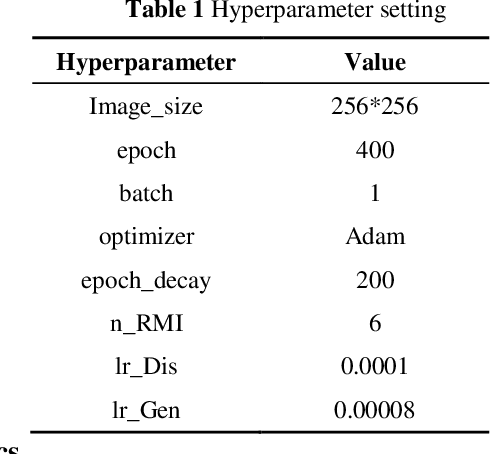
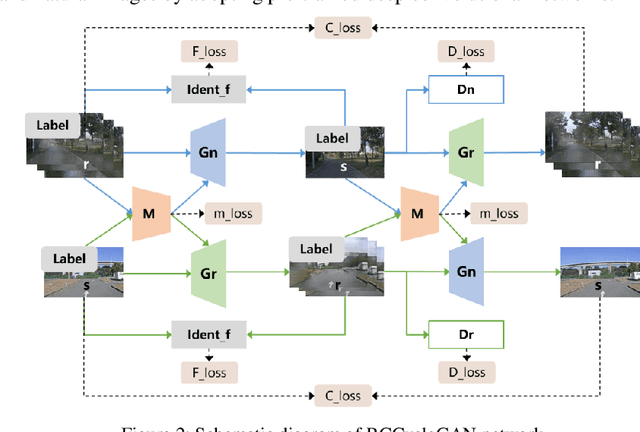
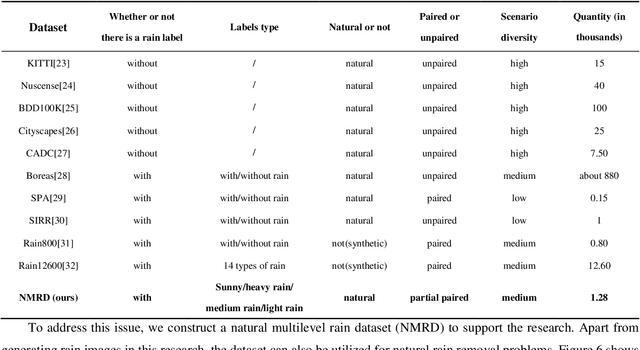
Abstract:Autonomous vehicles are exposed to various weather during operation, which is likely to trigger the performance limitations of the perception system, leading to the safety of the intended functionality (SOTIF) problems. To efficiently generate data for testing the performance of visual perception algorithms under various weather conditions, a hierarchical-level rain image generative model, rain conditional CycleGAN (RCCycleGAN), is constructed. RCCycleGAN is based on the generative adversarial network (GAN) and can generate images of light, medium, and heavy rain. Different rain intensities are introduced as labels in conditional GAN (CGAN). Meanwhile, the model structure is optimized and the training strategy is adjusted to alleviate the problem of mode collapse. In addition, natural rain images of different intensities are collected and processed for model training and validation. Compared with the two baseline models, CycleGAN and DerainCycleGAN, the peak signal-to-noise ratio (PSNR) of RCCycleGAN on the test dataset is improved by 2.58 dB and 0.74 dB, and the structural similarity (SSIM) is improved by 18% and 8%, respectively. The ablation experiments are also carried out to validate the effectiveness of the model tuning.
A Surface-Based Federated Chow Test Model for Integrating APOE Status, Tau Deposition Measure, and Hippocampal Surface Morphometry
Mar 31, 2023Abstract:Background: Alzheimer's Disease (AD) is the most common type of age-related dementia, affecting 6.2 million people aged 65 or older according to CDC data. It is commonly agreed that discovering an effective AD diagnosis biomarker could have enormous public health benefits, potentially preventing or delaying up to 40% of dementia cases. Tau neurofibrillary tangles are the primary driver of downstream neurodegeneration and subsequent cognitive impairment in AD, resulting in structural deformations such as hippocampal atrophy that can be observed in magnetic resonance imaging (MRI) scans. Objective: To build a surface-based model to 1) detect differences between APOE subgroups in patterns of tau deposition and hippocampal atrophy, and 2) use the extracted surface-based features to predict cognitive decline. Methods: Using data obtained from different institutions, we develop a surface-based federated Chow test model to study the synergistic effects of APOE, a previously reported significant risk factor of AD, and tau on hippocampal surface morphometry. Results: We illustrate that the APOE-specific morphometry features correlate with AD progression and better predict future AD conversion than other MRI biomarkers. For example, a strong association between atrophy and abnormal tau was identified in hippocampal subregion cornu ammonis 1 (CA1 subfield) and subiculum in e4 homozygote cohort. Conclusion: Our model allows for identifying MRI biomarkers for AD and cognitive decline prediction and may uncover a corner of the neural mechanism of the influence of APOE and tau deposition on hippocampal morphology.
Relation-dependent Contrastive Learning with Cluster Sampling for Inductive Relation Prediction
Nov 22, 2022



Abstract:Relation prediction is a task designed for knowledge graph completion which aims to predict missing relationships between entities. Recent subgraph-based models for inductive relation prediction have received increasing attention, which can predict relation for unseen entities based on the extracted subgraph surrounding the candidate triplet. However, they are not completely inductive because of their disability of predicting unseen relations. Moreover, they fail to pay sufficient attention to the role of relation as they only depend on the model to learn parameterized relation embedding, which leads to inaccurate prediction on long-tail relations. In this paper, we introduce Relation-dependent Contrastive Learning (ReCoLe) for inductive relation prediction, which adapts contrastive learning with a novel sampling method based on clustering algorithm to enhance the role of relation and improve the generalization ability to unseen relations. Instead of directly learning embedding for relations, ReCoLe allocates a pre-trained GNN-based encoder to each relation to strengthen the influence of relation. The GNN-based encoder is optimized by contrastive learning, which ensures satisfactory performance on long-tail relations. In addition, the cluster sampling method equips ReCoLe with the ability to handle both unseen relations and entities. Experimental results suggest that ReCoLe outperforms state-of-the-art methods on commonly used inductive datasets.
Improved Prediction of Beta-Amyloid and Tau Burden Using Hippocampal Surface Multivariate Morphometry Statistics and Sparse Coding
Oct 28, 2022



Abstract:Background: Beta-amyloid (A$\beta$) plaques and tau protein tangles in the brain are the defining 'A' and 'T' hallmarks of Alzheimer's disease (AD), and together with structural atrophy detectable on brain magnetic resonance imaging (MRI) scans as one of the neurodegenerative ('N') biomarkers comprise the ''ATN framework'' of AD. Current methods to detect A$\beta$/tau pathology include cerebrospinal fluid (CSF; invasive), positron emission tomography (PET; costly and not widely available), and blood-based biomarkers (BBBM; promising but mainly still in development). Objective: To develop a non-invasive and widely available structural MRI-based framework to quantitatively predict the amyloid and tau measurements. Methods: With MRI-based hippocampal multivariate morphometry statistics (MMS) features, we apply our Patch Analysis-based Surface Correntropy-induced Sparse coding and max-pooling (PASCS-MP) method combined with the ridge regression model to individual amyloid/tau measure prediction. Results: We evaluate our framework on amyloid PET/MRI and tau PET/MRI datasets from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Each subject has one pair consisting of a PET image and MRI scan, collected at about the same time. Experimental results suggest that amyloid/tau measurements predicted with our PASCP-MP representations are closer to the real values than the measures derived from other approaches, such as hippocampal surface area, volume, and shape morphometry features based on spherical harmonics (SPHARM). Conclusion: The MMS-based PASCP-MP is an efficient tool that can bridge hippocampal atrophy with amyloid and tau pathology and thus help assess disease burden, progression, and treatment effects.
Predicting Tau Accumulation in Cerebral Cortex with Multivariate MRI Morphometry Measurements, Sparse Coding, and Correntropy
Oct 20, 2021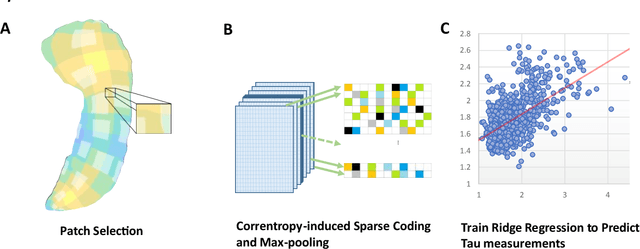
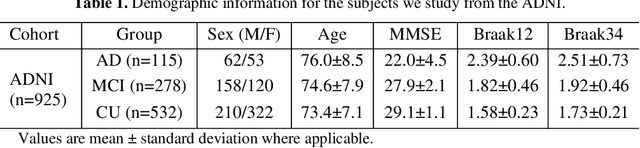
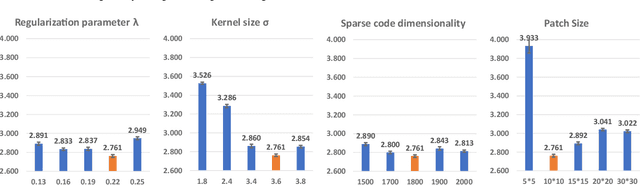
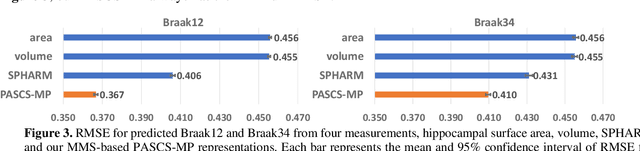
Abstract:Biomarker-assisted diagnosis and intervention in Alzheimer's disease (AD) may be the key to prevention breakthroughs. One of the hallmarks of AD is the accumulation of tau plaques in the human brain. However, current methods to detect tau pathology are either invasive (lumbar puncture) or quite costly and not widely available (Tau PET). In our previous work, structural MRI-based hippocampal multivariate morphometry statistics (MMS) showed superior performance as an effective neurodegenerative biomarker for preclinical AD and Patch Analysis-based Surface Correntropy-induced Sparse coding and max-pooling (PASCS-MP) has excellent ability to generate low-dimensional representations with strong statistical power for brain amyloid prediction. In this work, we apply this framework together with ridge regression models to predict Tau deposition in Braak12 and Braak34 brain regions separately. We evaluate our framework on 925 subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Each subject has one pair consisting of a PET image and MRI scan which were collected at about the same times. Experimental results suggest that the representations from our MMS and PASCS-MP have stronger predictive power and their predicted Braak12 and Braak34 are closer to the real values compared to the measures derived from other approaches such as hippocampal surface area and volume, and shape morphometry features based on spherical harmonics (SPHARM).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge