Wenhui Zhu
for the Alzheimer's Disease Neuroimaging Initiative
OTPrune: Distribution-Aligned Visual Token Pruning via Optimal Transport
Feb 25, 2026Abstract:Multi-modal large language models (MLLMs) achieve strong visual-language reasoning but suffer from high inference cost due to redundant visual tokens. Recent work explores visual token pruning to accelerate inference, while existing pruning methods overlook the underlying distributional structure of visual representations. We propose OTPrune, a training-free framework that formulates pruning as distribution alignment via optimal transport (OT). By minimizing the 2-Wasserstein distance between the full and pruned token distributions, OTPrune preserves both local diversity and global representativeness while reducing inference cost. Moreover, we derive a tractable submodular objective that enables efficient optimization, and theoretically prove its monotonicity and submodularity, providing a principled foundation for stable and efficient pruning. We further provide a comprehensive analysis that explains how distributional alignment contributes to stable and semantically faithful pruning. Comprehensive experiments on wider benchmarks demonstrate that OTPrune achieves superior performance-efficiency tradeoffs compared to state-of-the-art methods. The code is available at https://github.com/xiwenc1/OTPrune.
SGW-GAN: Sliced Gromov-Wasserstein Guided GANs for Retinal Fundus Image Enhancement
Jan 19, 2026Abstract:Retinal fundus photography is indispensable for ophthalmic screening and diagnosis, yet image quality is often degraded by noise, artifacts, and uneven illumination. Recent GAN- and diffusion-based enhancement methods improve perceptual quality by aligning degraded images with high-quality distributions, but our analysis shows that this focus can distort intra-class geometry: clinically related samples become dispersed, disease-class boundaries blur, and downstream tasks such as grading or lesion detection are harmed. The Gromov Wasserstein (GW) discrepancy offers a principled solution by aligning distributions through internal pairwise distances, naturally preserving intra-class structure, but its high computational cost restricts practical use. To overcome this, we propose SGW-GAN, the first framework to incorporate Sliced GW (SGW) into retinal image enhancement. SGW approximates GW via random projections, retaining relational fidelity while greatly reducing cost. Experiments on public datasets show that SGW-GAN produces visually compelling enhancements, achieves superior diabetic retinopathy grading, and reports the lowest GW discrepancy across disease labels, demonstrating both efficiency and clinical fidelity for unpaired medical image enhancement.
AHA: Aligning Large Audio-Language Models for Reasoning Hallucinations via Counterfactual Hard Negatives
Dec 30, 2025Abstract:Although Large Audio-Language Models (LALMs) deliver state-of-the-art (SOTA) performance, they frequently suffer from hallucinations, e.g. generating text not grounded in the audio input. We analyze these grounding failures and identify a distinct taxonomy: Event Omission, False Event Identity, Temporal Relation Error, and Quantitative Temporal Error. To address this, we introduce the AHA (Audio Hallucination Alignment) framework. By leveraging counterfactual hard negative mining, our pipeline constructs a high-quality preference dataset that forces models to distinguish strict acoustic evidence from linguistically plausible fabrications. Additionally, we establish AHA-Eval, a diagnostic benchmark designed to rigorously test these fine-grained temporal reasoning capabilities. We apply this data to align Qwen2.5-Omni. The resulting model, Qwen-Audio-AHA, achieves a 13.7% improvement on AHA-Eval. Crucially, this benefit generalizes beyond our diagnostic set. Our model shows substantial gains on public benchmarks, including 1.3% on MMAU-Test and 1.6% on MMAR, outperforming latest SOTA methods.
Fast 2DGS: Efficient Image Representation with Deep Gaussian Prior
Dec 14, 2025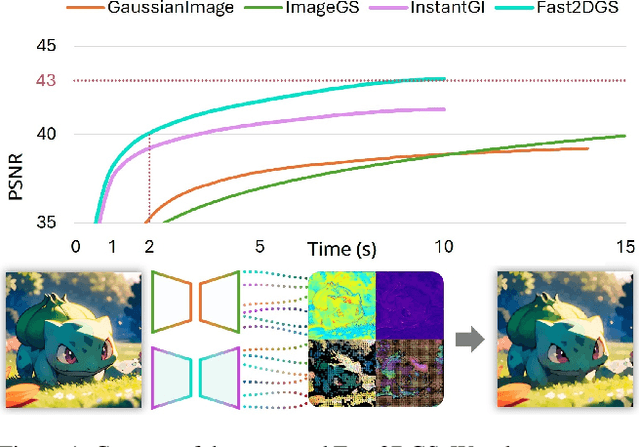
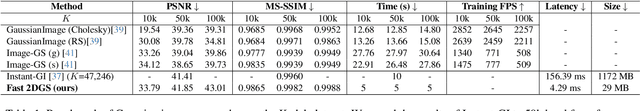
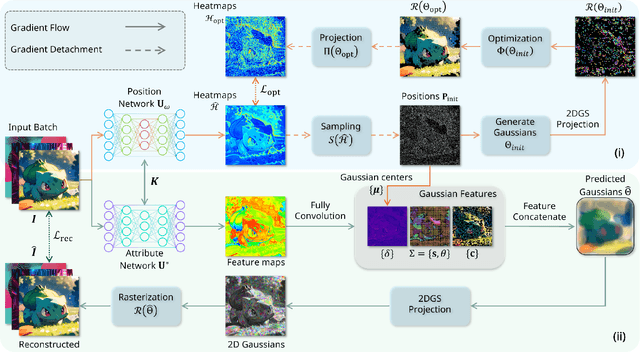
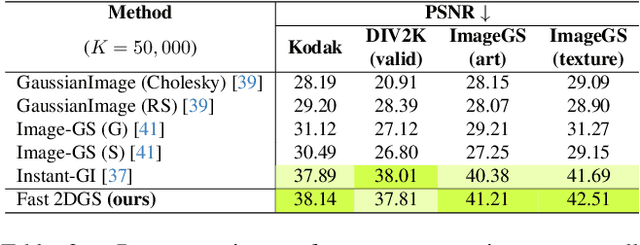
Abstract:As generative models become increasingly capable of producing high-fidelity visual content, the demand for efficient, interpretable, and editable image representations has grown substantially. Recent advances in 2D Gaussian Splatting (2DGS) have emerged as a promising solution, offering explicit control, high interpretability, and real-time rendering capabilities (>1000 FPS). However, high-quality 2DGS typically requires post-optimization. Existing methods adopt random or heuristics (e.g., gradient maps), which are often insensitive to image complexity and lead to slow convergence (>10s). More recent approaches introduce learnable networks to predict initial Gaussian configurations, but at the cost of increased computational and architectural complexity. To bridge this gap, we present Fast-2DGS, a lightweight framework for efficient Gaussian image representation. Specifically, we introduce Deep Gaussian Prior, implemented as a conditional network to capture the spatial distribution of Gaussian primitives under different complexities. In addition, we propose an attribute regression network to predict dense Gaussian properties. Experiments demonstrate that this disentangled architecture achieves high-quality reconstruction in a single forward pass, followed by minimal fine-tuning. More importantly, our approach significantly reduces computational cost without compromising visual quality, bringing 2DGS closer to industry-ready deployment.
Toward Effective Reinforcement Learning Fine-Tuning for Medical VQA in Vision-Language Models
May 20, 2025

Abstract:Recently, reinforcement learning (RL)-based tuning has shifted the trajectory of Multimodal Large Language Models (MLLMs), particularly following the introduction of Group Relative Policy Optimization (GRPO). However, directly applying it to medical tasks remains challenging for achieving clinically grounded model behavior. Motivated by the need to align model response with clinical expectations, we investigate four critical dimensions that affect the effectiveness of RL-based tuning in medical visual question answering (VQA): base model initialization strategy, the role of medical semantic alignment, the impact of length-based rewards on long-chain reasoning, and the influence of bias. We conduct extensive experiments to analyze these factors for medical MLLMs, providing new insights into how models are domain-specifically fine-tuned. Additionally, our results also demonstrate that GRPO-based RL tuning consistently outperforms standard supervised fine-tuning (SFT) in both accuracy and reasoning quality.
DRA-GRPO: Exploring Diversity-Aware Reward Adjustment for R1-Zero-Like Training of Large Language Models
May 14, 2025Abstract:Recent advances in reinforcement learning for language model post-training, such as Group Relative Policy Optimization (GRPO), have shown promise in low-resource settings. However, GRPO typically relies on solution-level and scalar reward signals that fail to capture the semantic diversity among sampled completions. This leads to what we identify as a diversity-quality inconsistency, where distinct reasoning paths may receive indistinguishable rewards. To address this limitation, we propose $\textit{Diversity-aware Reward Adjustment}$ (DRA), a method that explicitly incorporates semantic diversity into the reward computation. DRA uses Submodular Mutual Information (SMI) to downweight redundant completions and amplify rewards for diverse ones. This encourages better exploration during learning, while maintaining stable exploitation of high-quality samples. Our method integrates seamlessly with both GRPO and its variant DR.~GRPO, resulting in $\textit{DRA-GRPO}$ and $\textit{DGA-DR.~GRPO}$. We evaluate our method on five mathematical reasoning benchmarks and find that it outperforms recent strong baselines. It achieves state-of-the-art performance with an average accuracy of 58.2%, using only 7,000 fine-tuning samples and a total training cost of approximately $55. The code is available at https://github.com/xiwenc1/DRA-GRPO.
FIC-TSC: Learning Time Series Classification with Fisher Information Constraint
May 09, 2025



Abstract:Analyzing time series data is crucial to a wide spectrum of applications, including economics, online marketplaces, and human healthcare. In particular, time series classification plays an indispensable role in segmenting different phases in stock markets, predicting customer behavior, and classifying worker actions and engagement levels. These aspects contribute significantly to the advancement of automated decision-making and system optimization in real-world applications. However, there is a large consensus that time series data often suffers from domain shifts between training and test sets, which dramatically degrades the classification performance. Despite the success of (reversible) instance normalization in handling the domain shifts for time series regression tasks, its performance in classification is unsatisfactory. In this paper, we propose \textit{FIC-TSC}, a training framework for time series classification that leverages Fisher information as the constraint. We theoretically and empirically show this is an efficient and effective solution to guide the model converge toward flatter minima, which enhances its generalizability to distribution shifts. We rigorously evaluate our method on 30 UEA multivariate and 85 UCR univariate datasets. Our empirical results demonstrate the superiority of the proposed method over 14 recent state-of-the-art methods.
Talk Before You Retrieve: Agent-Led Discussions for Better RAG in Medical QA
Apr 30, 2025Abstract:Medical question answering (QA) is a reasoning-intensive task that remains challenging for large language models (LLMs) due to hallucinations and outdated domain knowledge. Retrieval-Augmented Generation (RAG) provides a promising post-training solution by leveraging external knowledge. However, existing medical RAG systems suffer from two key limitations: (1) a lack of modeling for human-like reasoning behaviors during information retrieval, and (2) reliance on suboptimal medical corpora, which often results in the retrieval of irrelevant or noisy snippets. To overcome these challenges, we propose Discuss-RAG, a plug-and-play module designed to enhance the medical QA RAG system through collaborative agent-based reasoning. Our method introduces a summarizer agent that orchestrates a team of medical experts to emulate multi-turn brainstorming, thereby improving the relevance of retrieved content. Additionally, a decision-making agent evaluates the retrieved snippets before their final integration. Experimental results on four benchmark medical QA datasets show that Discuss-RAG consistently outperforms MedRAG, especially significantly improving answer accuracy by up to 16.67% on BioASQ and 12.20% on PubMedQA. The code is available at: https://github.com/LLM-VLM-GSL/Discuss-RAG.
A BERT-Style Self-Supervised Learning CNN for Disease Identification from Retinal Images
Apr 25, 2025Abstract:In the field of medical imaging, the advent of deep learning, especially the application of convolutional neural networks (CNNs) has revolutionized the analysis and interpretation of medical images. Nevertheless, deep learning methods usually rely on large amounts of labeled data. In medical imaging research, the acquisition of high-quality labels is both expensive and difficult. The introduction of Vision Transformers (ViT) and self-supervised learning provides a pre-training strategy that utilizes abundant unlabeled data, effectively alleviating the label acquisition challenge while broadening the breadth of data utilization. However, ViT's high computational density and substantial demand for computing power, coupled with the lack of localization characteristics of its operations on image patches, limit its efficiency and applicability in many application scenarios. In this study, we employ nn-MobileNet, a lightweight CNN framework, to implement a BERT-style self-supervised learning approach. We pre-train the network on the unlabeled retinal fundus images from the UK Biobank to improve downstream application performance. We validate the results of the pre-trained model on Alzheimer's disease (AD), Parkinson's disease (PD), and various retinal diseases identification. The results show that our approach can significantly improve performance in the downstream tasks. In summary, this study combines the benefits of CNNs with the capabilities of advanced self-supervised learning in handling large-scale unlabeled data, demonstrating the potential of CNNs in the presence of label scarcity.
How Effective Can Dropout Be in Multiple Instance Learning ?
Apr 21, 2025Abstract:Multiple Instance Learning (MIL) is a popular weakly-supervised method for various applications, with a particular interest in histological whole slide image (WSI) classification. Due to the gigapixel resolution of WSI, applications of MIL in WSI typically necessitate a two-stage training scheme: first, extract features from the pre-trained backbone and then perform MIL aggregation. However, it is well-known that this suboptimal training scheme suffers from "noisy" feature embeddings from the backbone and inherent weak supervision, hindering MIL from learning rich and generalizable features. However, the most commonly used technique (i.e., dropout) for mitigating this issue has yet to be explored in MIL. In this paper, we empirically explore how effective the dropout can be in MIL. Interestingly, we observe that dropping the top-k most important instances within a bag leads to better performance and generalization even under noise attack. Based on this key observation, we propose a novel MIL-specific dropout method, termed MIL-Dropout, which systematically determines which instances to drop. Experiments on five MIL benchmark datasets and two WSI datasets demonstrate that MIL-Dropout boosts the performance of current MIL methods with a negligible computational cost. The code is available at https://github.com/ChongQingNoSubway/MILDropout.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge