Xuanzhao Dong
SGW-GAN: Sliced Gromov-Wasserstein Guided GANs for Retinal Fundus Image Enhancement
Jan 19, 2026Abstract:Retinal fundus photography is indispensable for ophthalmic screening and diagnosis, yet image quality is often degraded by noise, artifacts, and uneven illumination. Recent GAN- and diffusion-based enhancement methods improve perceptual quality by aligning degraded images with high-quality distributions, but our analysis shows that this focus can distort intra-class geometry: clinically related samples become dispersed, disease-class boundaries blur, and downstream tasks such as grading or lesion detection are harmed. The Gromov Wasserstein (GW) discrepancy offers a principled solution by aligning distributions through internal pairwise distances, naturally preserving intra-class structure, but its high computational cost restricts practical use. To overcome this, we propose SGW-GAN, the first framework to incorporate Sliced GW (SGW) into retinal image enhancement. SGW approximates GW via random projections, retaining relational fidelity while greatly reducing cost. Experiments on public datasets show that SGW-GAN produces visually compelling enhancements, achieves superior diabetic retinopathy grading, and reports the lowest GW discrepancy across disease labels, demonstrating both efficiency and clinical fidelity for unpaired medical image enhancement.
AHA: Aligning Large Audio-Language Models for Reasoning Hallucinations via Counterfactual Hard Negatives
Dec 30, 2025Abstract:Although Large Audio-Language Models (LALMs) deliver state-of-the-art (SOTA) performance, they frequently suffer from hallucinations, e.g. generating text not grounded in the audio input. We analyze these grounding failures and identify a distinct taxonomy: Event Omission, False Event Identity, Temporal Relation Error, and Quantitative Temporal Error. To address this, we introduce the AHA (Audio Hallucination Alignment) framework. By leveraging counterfactual hard negative mining, our pipeline constructs a high-quality preference dataset that forces models to distinguish strict acoustic evidence from linguistically plausible fabrications. Additionally, we establish AHA-Eval, a diagnostic benchmark designed to rigorously test these fine-grained temporal reasoning capabilities. We apply this data to align Qwen2.5-Omni. The resulting model, Qwen-Audio-AHA, achieves a 13.7% improvement on AHA-Eval. Crucially, this benefit generalizes beyond our diagnostic set. Our model shows substantial gains on public benchmarks, including 1.3% on MMAU-Test and 1.6% on MMAR, outperforming latest SOTA methods.
Fast 2DGS: Efficient Image Representation with Deep Gaussian Prior
Dec 14, 2025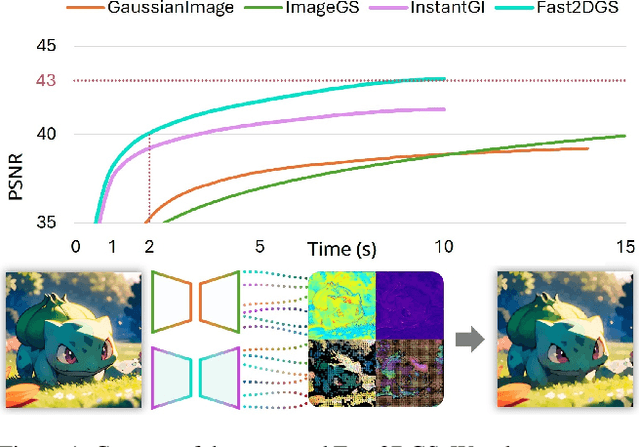
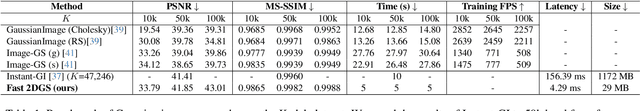
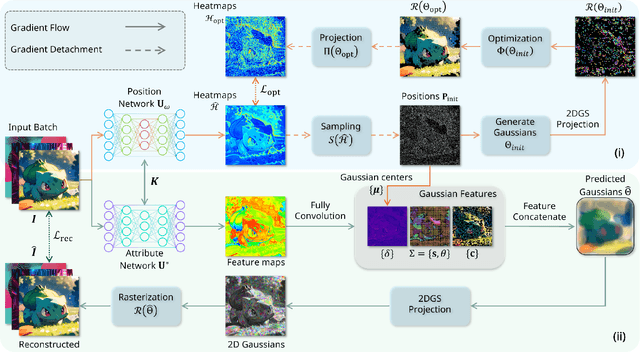
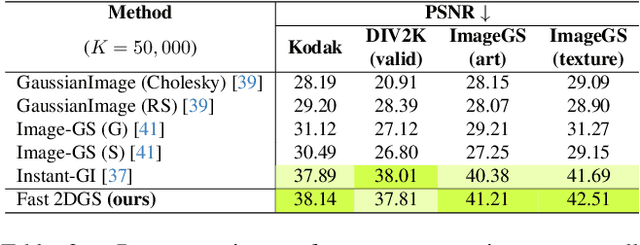
Abstract:As generative models become increasingly capable of producing high-fidelity visual content, the demand for efficient, interpretable, and editable image representations has grown substantially. Recent advances in 2D Gaussian Splatting (2DGS) have emerged as a promising solution, offering explicit control, high interpretability, and real-time rendering capabilities (>1000 FPS). However, high-quality 2DGS typically requires post-optimization. Existing methods adopt random or heuristics (e.g., gradient maps), which are often insensitive to image complexity and lead to slow convergence (>10s). More recent approaches introduce learnable networks to predict initial Gaussian configurations, but at the cost of increased computational and architectural complexity. To bridge this gap, we present Fast-2DGS, a lightweight framework for efficient Gaussian image representation. Specifically, we introduce Deep Gaussian Prior, implemented as a conditional network to capture the spatial distribution of Gaussian primitives under different complexities. In addition, we propose an attribute regression network to predict dense Gaussian properties. Experiments demonstrate that this disentangled architecture achieves high-quality reconstruction in a single forward pass, followed by minimal fine-tuning. More importantly, our approach significantly reduces computational cost without compromising visual quality, bringing 2DGS closer to industry-ready deployment.
Toward Effective Reinforcement Learning Fine-Tuning for Medical VQA in Vision-Language Models
May 20, 2025

Abstract:Recently, reinforcement learning (RL)-based tuning has shifted the trajectory of Multimodal Large Language Models (MLLMs), particularly following the introduction of Group Relative Policy Optimization (GRPO). However, directly applying it to medical tasks remains challenging for achieving clinically grounded model behavior. Motivated by the need to align model response with clinical expectations, we investigate four critical dimensions that affect the effectiveness of RL-based tuning in medical visual question answering (VQA): base model initialization strategy, the role of medical semantic alignment, the impact of length-based rewards on long-chain reasoning, and the influence of bias. We conduct extensive experiments to analyze these factors for medical MLLMs, providing new insights into how models are domain-specifically fine-tuned. Additionally, our results also demonstrate that GRPO-based RL tuning consistently outperforms standard supervised fine-tuning (SFT) in both accuracy and reasoning quality.
DRA-GRPO: Exploring Diversity-Aware Reward Adjustment for R1-Zero-Like Training of Large Language Models
May 14, 2025Abstract:Recent advances in reinforcement learning for language model post-training, such as Group Relative Policy Optimization (GRPO), have shown promise in low-resource settings. However, GRPO typically relies on solution-level and scalar reward signals that fail to capture the semantic diversity among sampled completions. This leads to what we identify as a diversity-quality inconsistency, where distinct reasoning paths may receive indistinguishable rewards. To address this limitation, we propose $\textit{Diversity-aware Reward Adjustment}$ (DRA), a method that explicitly incorporates semantic diversity into the reward computation. DRA uses Submodular Mutual Information (SMI) to downweight redundant completions and amplify rewards for diverse ones. This encourages better exploration during learning, while maintaining stable exploitation of high-quality samples. Our method integrates seamlessly with both GRPO and its variant DR.~GRPO, resulting in $\textit{DRA-GRPO}$ and $\textit{DGA-DR.~GRPO}$. We evaluate our method on five mathematical reasoning benchmarks and find that it outperforms recent strong baselines. It achieves state-of-the-art performance with an average accuracy of 58.2%, using only 7,000 fine-tuning samples and a total training cost of approximately $55. The code is available at https://github.com/xiwenc1/DRA-GRPO.
Talk Before You Retrieve: Agent-Led Discussions for Better RAG in Medical QA
Apr 30, 2025Abstract:Medical question answering (QA) is a reasoning-intensive task that remains challenging for large language models (LLMs) due to hallucinations and outdated domain knowledge. Retrieval-Augmented Generation (RAG) provides a promising post-training solution by leveraging external knowledge. However, existing medical RAG systems suffer from two key limitations: (1) a lack of modeling for human-like reasoning behaviors during information retrieval, and (2) reliance on suboptimal medical corpora, which often results in the retrieval of irrelevant or noisy snippets. To overcome these challenges, we propose Discuss-RAG, a plug-and-play module designed to enhance the medical QA RAG system through collaborative agent-based reasoning. Our method introduces a summarizer agent that orchestrates a team of medical experts to emulate multi-turn brainstorming, thereby improving the relevance of retrieved content. Additionally, a decision-making agent evaluates the retrieved snippets before their final integration. Experimental results on four benchmark medical QA datasets show that Discuss-RAG consistently outperforms MedRAG, especially significantly improving answer accuracy by up to 16.67% on BioASQ and 12.20% on PubMedQA. The code is available at: https://github.com/LLM-VLM-GSL/Discuss-RAG.
Enhancing 3T BOLD fMRI SNR using Unpaired 7T Data with Schrödinger Bridge Diffusion
Apr 01, 2025Abstract:High spatial and temporal resolution, coupled with a strong signal-to-noise ratio (SNR), has made BOLD 7 Tesla fMRI an invaluable tool for understanding how the brain processes visual stimuli. However, the limited availability of 7T MRI systems means that most research relies on 3T MRI systems, which offer lower spatial and temporal resolution and SNR. This naturally raises the question: Can we enhance the spatiotemporal resolution and SNR of 3T BOLD fMRI data to approximate 7T quality? In this study, we propose a novel framework that aligns 7T and 3T fMRI data from different subjects and datasets in a shared parametric domain. We then apply an unpaired Brain Disk Schr\"odinger Bridge diffusion model to enhance the spatiotemporal resolution and SNR of the 3T data. Our approach addresses the challenge of limited 7T data by improving the 3T scan quality. We demonstrate its effectiveness by testing it on two distinct fMRI retinotopy datasets (one 7T and one 3T), as well as synthetic data. The results show that our method significantly improves the SNR and goodness-of-fit of the population receptive field (pRF) model in the enhanced 3T data, making it comparable to 7T quality. The codes will be available at Github.
RetinalGPT: A Retinal Clinical Preference Conversational Assistant Powered by Large Vision-Language Models
Mar 06, 2025Abstract:Recently, Multimodal Large Language Models (MLLMs) have gained significant attention for their remarkable ability to process and analyze non-textual data, such as images, videos, and audio. Notably, several adaptations of general-domain MLLMs to the medical field have been explored, including LLaVA-Med. However, these medical adaptations remain insufficiently advanced in understanding and interpreting retinal images. In contrast, medical experts emphasize the importance of quantitative analyses for disease detection and interpretation. This underscores a gap between general-domain and medical-domain MLLMs: while general-domain MLLMs excel in broad applications, they lack the specialized knowledge necessary for precise diagnostic and interpretative tasks in the medical field. To address these challenges, we introduce \textit{RetinalGPT}, a multimodal conversational assistant for clinically preferred quantitative analysis of retinal images. Specifically, we achieve this by compiling a large retinal image dataset, developing a novel data pipeline, and employing customized visual instruction tuning to enhance both retinal analysis and enrich medical knowledge. In particular, RetinalGPT outperforms MLLM in the generic domain by a large margin in the diagnosis of retinal diseases in 8 benchmark retinal datasets. Beyond disease diagnosis, RetinalGPT features quantitative analyses and lesion localization, representing a pioneering step in leveraging LLMs for an interpretable and end-to-end clinical research framework. The code is available at https://github.com/Retinal-Research/RetinalGPT
EyeBench: A Call for More Rigorous Evaluation of Retinal Image Enhancement
Feb 20, 2025



Abstract:Over the past decade, generative models have achieved significant success in enhancement fundus images.However, the evaluation of these models still presents a considerable challenge. A comprehensive evaluation benchmark for fundus image enhancement is indispensable for three main reasons: 1) The existing denoising metrics (e.g., PSNR, SSIM) are hardly to extend to downstream real-world clinical research (e.g., Vessel morphology consistency). 2) There is a lack of comprehensive evaluation for both paired and unpaired enhancement methods, along with the need for expert protocols to accurately assess clinical value. 3) An ideal evaluation system should provide insights to inform future developments of fundus image enhancement. To this end, we propose a novel comprehensive benchmark, EyeBench, to provide insights that align enhancement models with clinical needs, offering a foundation for future work to improve the clinical relevance and applicability of generative models for fundus image enhancement. EyeBench has three appealing properties: 1) multi-dimensional clinical alignment downstream evaluation: In addition to evaluating the enhancement task, we provide several clinically significant downstream tasks for fundus images, including vessel segmentation, DR grading, denoising generalization, and lesion segmentation. 2) Medical expert-guided evaluation design: We introduce a novel dataset that promote comprehensive and fair comparisons between paired and unpaired methods and includes a manual evaluation protocol by medical experts. 3) Valuable insights: Our benchmark study provides a comprehensive and rigorous evaluation of existing methods across different downstream tasks, assisting medical experts in making informed choices. Additionally, we offer further analysis of the challenges faced by existing methods. The code is available at \url{https://github.com/Retinal-Research/EyeBench}
Many-MobileNet: Multi-Model Augmentation for Robust Retinal Disease Classification
Dec 03, 2024



Abstract:In this work, we propose Many-MobileNet, an efficient model fusion strategy for retinal disease classification using lightweight CNN architecture. Our method addresses key challenges such as overfitting and limited dataset variability by training multiple models with distinct data augmentation strategies and different model complexities. Through this fusion technique, we achieved robust generalization in data-scarce domains while balancing computational efficiency with feature extraction capabilities.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge