Kewei Chen
for the Alzheimer's Disease Neuroimaging Initiative
Plasma-CycleGAN: Plasma Biomarker-Guided MRI to PET Cross-modality Translation Using Conditional CycleGAN
Jan 04, 2025



Abstract:Cross-modality translation between MRI and PET imaging is challenging due to the distinct mechanisms underlying these modalities. Blood-based biomarkers (BBBMs) are revolutionizing Alzheimer's disease (AD) detection by identifying patients and quantifying brain amyloid levels. However, the potential of BBBMs to enhance PET image synthesis remains unexplored. In this paper, we performed a thorough study on the effect of incorporating BBBM into deep generative models. By evaluating three widely used cross-modality translation models, we found that BBBMs integration consistently enhances the generative quality across all models. By visual inspection of the generated results, we observed that PET images generated by CycleGAN exhibit the best visual fidelity. Based on these findings, we propose Plasma-CycleGAN, a novel generative model based on CycleGAN, to synthesize PET images from MRI using BBBMs as conditions. This is the first approach to integrate BBBMs in conditional cross-modality translation between MRI and PET.
STEVE Series: Step-by-Step Construction of Agent Systems in Minecraft
Jun 17, 2024Abstract:Building an embodied agent system with a large language model (LLM) as its core is a promising direction. Due to the significant costs and uncontrollable factors associated with deploying and training such agents in the real world, we have decided to begin our exploration within the Minecraft environment. Our STEVE Series agents can complete basic tasks in a virtual environment and more challenging tasks such as navigation and even creative tasks, with an efficiency far exceeding previous state-of-the-art methods by a factor of $2.5\times$ to $7.3\times$. We begin our exploration with a vanilla large language model, augmenting it with a vision encoder and an action codebase trained on our collected high-quality dataset STEVE-21K. Subsequently, we enhanced it with a Critic and memory to transform it into a complex system. Finally, we constructed a hierarchical multi-agent system. Our recent work explored how to prune the agent system through knowledge distillation. In the future, we will explore more potential applications of STEVE agents in the real world.
Do We Really Need a Complex Agent System? Distill Embodied Agent into a Single Model
Apr 06, 2024Abstract:With the power of large language models (LLMs), open-ended embodied agents can flexibly understand human instructions, generate interpretable guidance strategies, and output executable actions. Nowadays, Multi-modal Language Models~(MLMs) integrate multi-modal signals into LLMs, further bringing richer perception to entity agents and allowing embodied agents to perceive world-understanding tasks more delicately. However, existing works: 1) operate independently by agents, each containing multiple LLMs, from perception to action, resulting in gaps between complex tasks and execution; 2) train MLMs on static data, struggling with dynamics in open-ended scenarios; 3) input prior knowledge directly as prompts, suppressing application flexibility. We propose STEVE-2, a hierarchical knowledge distillation framework for open-ended embodied tasks, characterized by 1) a hierarchical system for multi-granular task division, 2) a mirrored distillation method for parallel simulation data, and 3) an extra expert model for bringing additional knowledge into parallel simulation. After distillation, embodied agents can complete complex, open-ended tasks without additional expert guidance, utilizing the performance and knowledge of a versatile MLM. Extensive evaluations on navigation and creation tasks highlight the superior performance of STEVE-2 in open-ended tasks, with $1.4 \times$ - $7.3 \times$ in performance.
Hierarchical Auto-Organizing System for Open-Ended Multi-Agent Navigation
Mar 18, 2024Abstract:Due to the dynamic and unpredictable open-world setting, navigating complex environments in Minecraft poses significant challenges for multi-agent systems. Agents must interact with the environment and coordinate their actions with other agents to achieve common objectives. However, traditional approaches often struggle to efficiently manage inter-agent communication and task distribution, crucial for effective multi-agent navigation. Furthermore, processing and integrating multi-modal information (such as visual, textual, and auditory data) is essential for agents to comprehend their goals and navigate the environment successfully and fully. To address this issue, we design the HAS framework to auto-organize groups of LLM-based agents to complete navigation tasks. In our approach, we devise a hierarchical auto-organizing navigation system, which is characterized by 1) a hierarchical system for multi-agent organization, ensuring centralized planning and decentralized execution; 2) an auto-organizing and intra-communication mechanism, enabling dynamic group adjustment under subtasks; 3) a multi-modal information platform, facilitating multi-modal perception to perform the three navigation tasks with one system. To assess organizational behavior, we design a series of navigation tasks in the Minecraft environment, which includes searching and exploring. We aim to develop embodied organizations that push the boundaries of embodied AI, moving it towards a more human-like organizational structure.
A Surface-Based Federated Chow Test Model for Integrating APOE Status, Tau Deposition Measure, and Hippocampal Surface Morphometry
Mar 31, 2023Abstract:Background: Alzheimer's Disease (AD) is the most common type of age-related dementia, affecting 6.2 million people aged 65 or older according to CDC data. It is commonly agreed that discovering an effective AD diagnosis biomarker could have enormous public health benefits, potentially preventing or delaying up to 40% of dementia cases. Tau neurofibrillary tangles are the primary driver of downstream neurodegeneration and subsequent cognitive impairment in AD, resulting in structural deformations such as hippocampal atrophy that can be observed in magnetic resonance imaging (MRI) scans. Objective: To build a surface-based model to 1) detect differences between APOE subgroups in patterns of tau deposition and hippocampal atrophy, and 2) use the extracted surface-based features to predict cognitive decline. Methods: Using data obtained from different institutions, we develop a surface-based federated Chow test model to study the synergistic effects of APOE, a previously reported significant risk factor of AD, and tau on hippocampal surface morphometry. Results: We illustrate that the APOE-specific morphometry features correlate with AD progression and better predict future AD conversion than other MRI biomarkers. For example, a strong association between atrophy and abnormal tau was identified in hippocampal subregion cornu ammonis 1 (CA1 subfield) and subiculum in e4 homozygote cohort. Conclusion: Our model allows for identifying MRI biomarkers for AD and cognitive decline prediction and may uncover a corner of the neural mechanism of the influence of APOE and tau deposition on hippocampal morphology.
Improved Prediction of Beta-Amyloid and Tau Burden Using Hippocampal Surface Multivariate Morphometry Statistics and Sparse Coding
Oct 28, 2022



Abstract:Background: Beta-amyloid (A$\beta$) plaques and tau protein tangles in the brain are the defining 'A' and 'T' hallmarks of Alzheimer's disease (AD), and together with structural atrophy detectable on brain magnetic resonance imaging (MRI) scans as one of the neurodegenerative ('N') biomarkers comprise the ''ATN framework'' of AD. Current methods to detect A$\beta$/tau pathology include cerebrospinal fluid (CSF; invasive), positron emission tomography (PET; costly and not widely available), and blood-based biomarkers (BBBM; promising but mainly still in development). Objective: To develop a non-invasive and widely available structural MRI-based framework to quantitatively predict the amyloid and tau measurements. Methods: With MRI-based hippocampal multivariate morphometry statistics (MMS) features, we apply our Patch Analysis-based Surface Correntropy-induced Sparse coding and max-pooling (PASCS-MP) method combined with the ridge regression model to individual amyloid/tau measure prediction. Results: We evaluate our framework on amyloid PET/MRI and tau PET/MRI datasets from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Each subject has one pair consisting of a PET image and MRI scan, collected at about the same time. Experimental results suggest that amyloid/tau measurements predicted with our PASCP-MP representations are closer to the real values than the measures derived from other approaches, such as hippocampal surface area, volume, and shape morphometry features based on spherical harmonics (SPHARM). Conclusion: The MMS-based PASCP-MP is an efficient tool that can bridge hippocampal atrophy with amyloid and tau pathology and thus help assess disease burden, progression, and treatment effects.
Application of Machine Learning Methods in Inferring Surface Water Groundwater Exchanges using High Temporal Resolution Temperature Measurements
Jan 03, 2022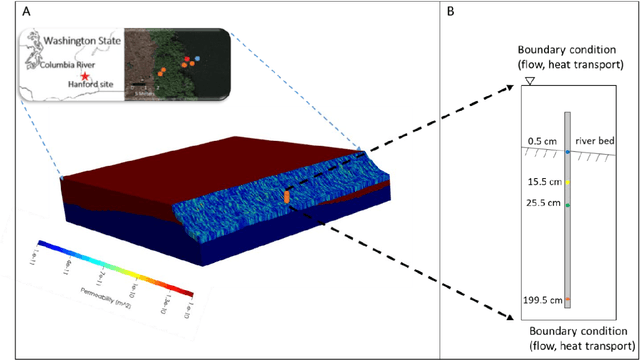
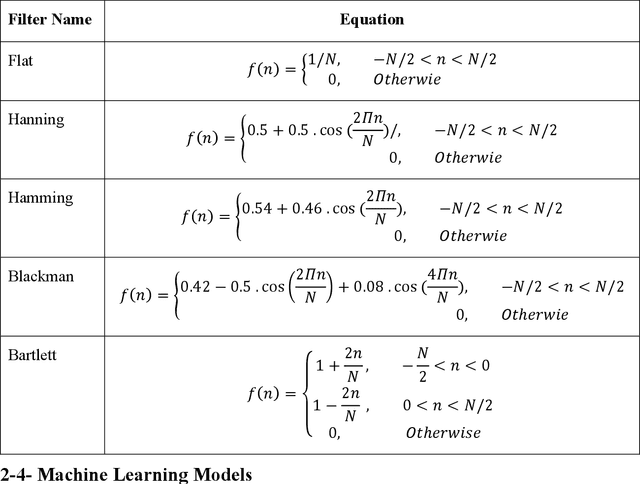
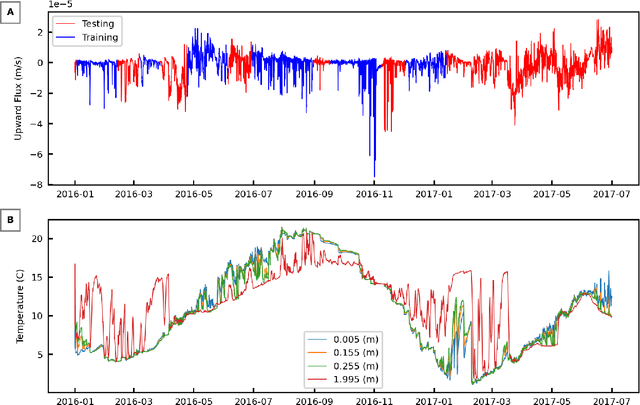
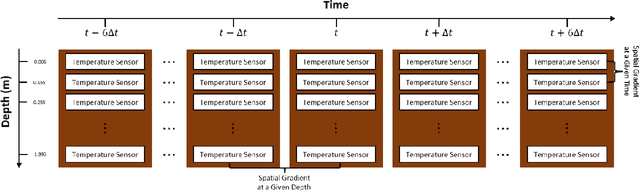
Abstract:We examine the ability of machine learning (ML) and deep learning (DL) algorithms to infer surface/ground exchange flux based on subsurface temperature observations. The observations and fluxes are produced from a high-resolution numerical model representing conditions in the Columbia River near the Department of Energy Hanford site located in southeastern Washington State. Random measurement error, of varying magnitude, is added to the synthetic temperature observations. The results indicate that both ML and DL methods can be used to infer the surface/ground exchange flux. DL methods, especially convolutional neural networks, outperform the ML methods when used to interpret noisy temperature data with a smoothing filter applied. However, the ML methods also performed well and they are can better identify a reduced number of important observations, which could be useful for measurement network optimization. Surprisingly, the ML and DL methods better inferred upward flux than downward flux. This is in direct contrast to previous findings using numerical models to infer flux from temperature observations and it may suggest that combined use of ML or DL inference with numerical inference could improve flux estimation beneath river systems.
Predicting Tau Accumulation in Cerebral Cortex with Multivariate MRI Morphometry Measurements, Sparse Coding, and Correntropy
Oct 20, 2021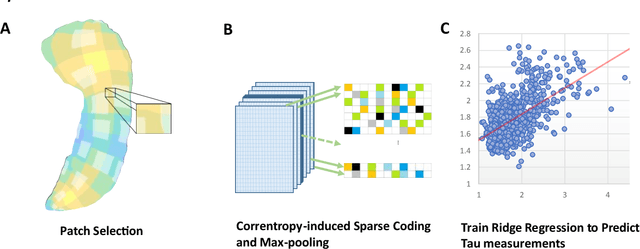
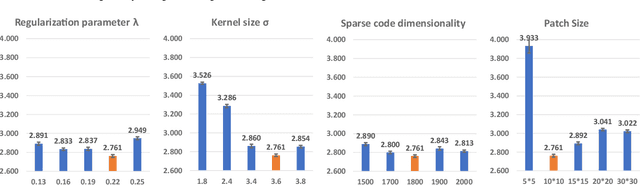
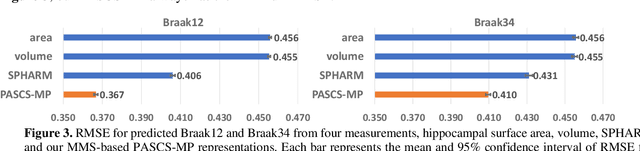
Abstract:Biomarker-assisted diagnosis and intervention in Alzheimer's disease (AD) may be the key to prevention breakthroughs. One of the hallmarks of AD is the accumulation of tau plaques in the human brain. However, current methods to detect tau pathology are either invasive (lumbar puncture) or quite costly and not widely available (Tau PET). In our previous work, structural MRI-based hippocampal multivariate morphometry statistics (MMS) showed superior performance as an effective neurodegenerative biomarker for preclinical AD and Patch Analysis-based Surface Correntropy-induced Sparse coding and max-pooling (PASCS-MP) has excellent ability to generate low-dimensional representations with strong statistical power for brain amyloid prediction. In this work, we apply this framework together with ridge regression models to predict Tau deposition in Braak12 and Braak34 brain regions separately. We evaluate our framework on 925 subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Each subject has one pair consisting of a PET image and MRI scan which were collected at about the same times. Experimental results suggest that the representations from our MMS and PASCS-MP have stronger predictive power and their predicted Braak12 and Braak34 are closer to the real values compared to the measures derived from other approaches such as hippocampal surface area and volume, and shape morphometry features based on spherical harmonics (SPHARM).
Developing Univariate Neurodegeneration Biomarkers with Low-Rank and Sparse Subspace Decomposition
Oct 26, 2020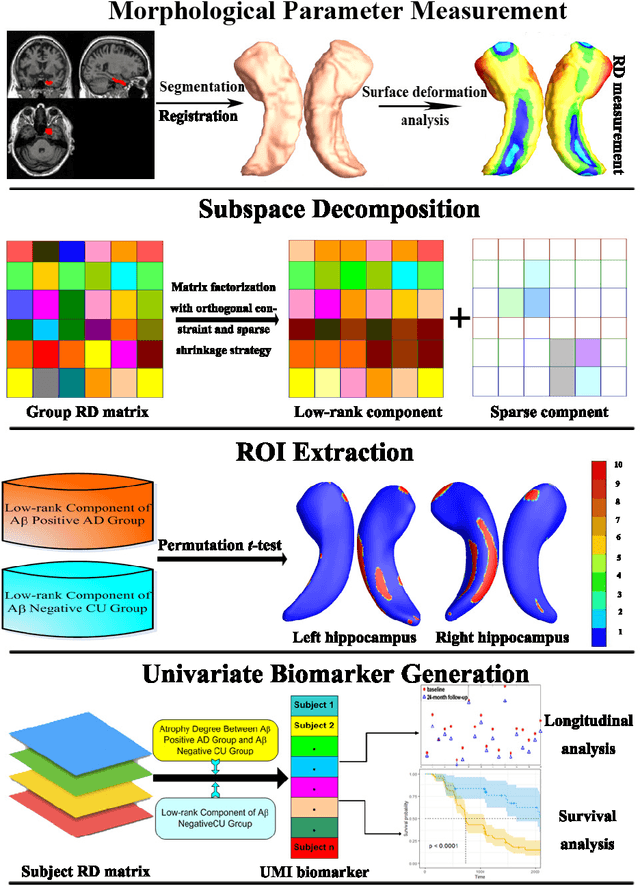
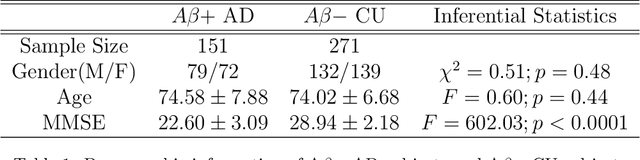
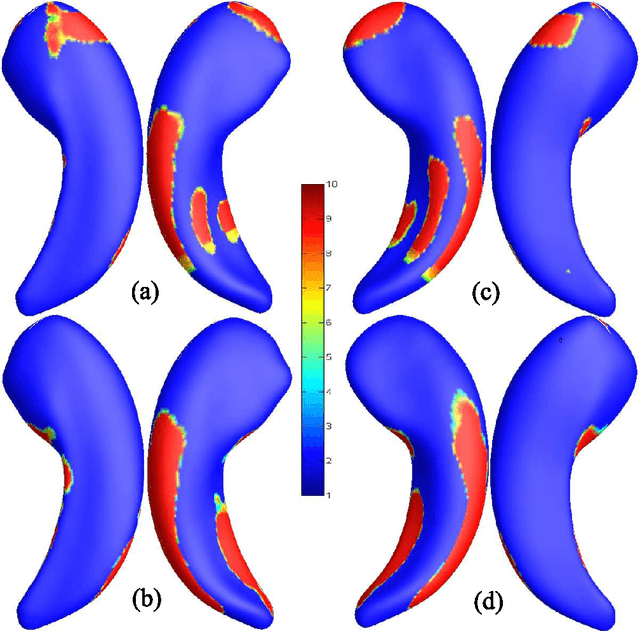
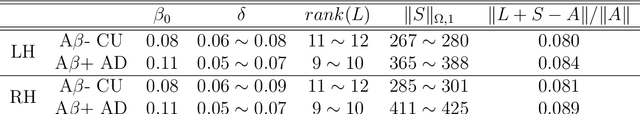
Abstract:Cognitive decline due to Alzheimer's disease (AD) is closely associated with brain structure alterations captured by structural magnetic resonance imaging (sMRI). It supports the validity to develop sMRI-based univariate neurodegeneration biomarkers (UNB). However, existing UNB work either fails to model large group variances or does not capture AD dementia (ADD) induced changes. We propose a novel low-rank and sparse subspace decomposition method capable of stably quantifying the morphological changes induced by ADD. Specifically, we propose a numerically efficient rank minimization mechanism to extract group common structure and impose regularization constraints to encode the original 3D morphometry connectivity. Further, we generate regions-of-interest (ROI) with group difference study between common subspaces of $A\beta+$ AD and $A\beta-$ cognitively unimpaired (CU) groups. A univariate morphometry index (UMI) is constructed from these ROIs by summarizing individual morphological characteristics weighted by normalized difference between $A\beta+$ AD and $A\beta-$ CU groups. We use hippocampal surface radial distance feature to compute the UMIs and validate our work in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. With hippocampal UMIs, the estimated minimum sample sizes needed to detect a 25$\%$ reduction in the mean annual change with 80$\%$ power and two-tailed $P=0.05$ are 116, 279 and 387 for the longitudinal $A\beta+$ AD, $A\beta+$ mild cognitive impairment (MCI) and $A\beta+$ CU groups, respectively. Additionally, for MCI patients, UMIs well correlate with hazard ratio of conversion to AD ($4.3$, $95\%$ CI=$2.3-8.2$) within 18 months. Our experimental results outperform traditional hippocampal volume measures and suggest the application of UMI as a potential UNB.
Self-PU: Self Boosted and Calibrated Positive-Unlabeled Training
Jun 22, 2020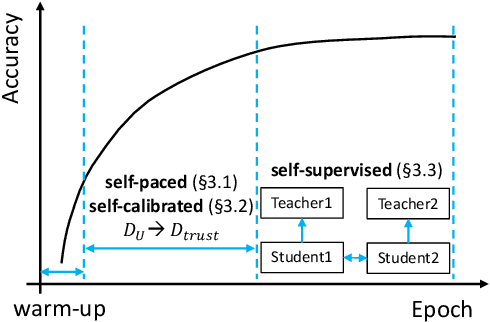
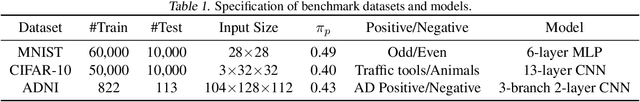
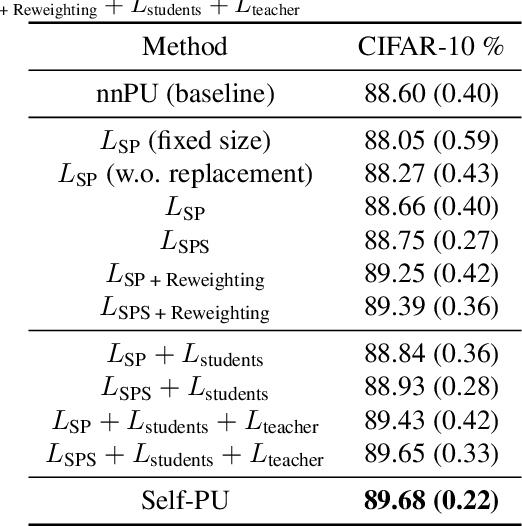
Abstract:Many real-world applications have to tackle the Positive-Unlabeled (PU) learning problem, i.e., learning binary classifiers from a large amount of unlabeled data and a few labeled positive examples. While current state-of-the-art methods employ importance reweighting to design various risk estimators, they ignored the learning capability of the model itself, which could have provided reliable supervision. This motivates us to propose a novel Self-PU learning framework, which seamlessly integrates PU learning and self-training. Self-PU highlights three "self"-oriented building blocks: a self-paced training algorithm that adaptively discovers and augments confident positive/negative examples as the training proceeds; a self-calibrated instance-aware loss; and a self-distillation scheme that introduces teacher-students learning as an effective regularization for PU learning. We demonstrate the state-of-the-art performance of Self-PU on common PU learning benchmarks (MNIST and CIFAR-10), which compare favorably against the latest competitors. Moreover, we study a real-world application of PU learning, i.e., classifying brain images of Alzheimer's Disease. Self-PU obtains significantly improved results on the renowned Alzheimer's Disease Neuroimaging Initiative (ADNI) database over existing methods. The code is publicly available at: https://github.com/TAMU-VITA/Self-PU.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge