Richard J Caselli
Plasma-CycleGAN: Plasma Biomarker-Guided MRI to PET Cross-modality Translation Using Conditional CycleGAN
Jan 04, 2025



Abstract:Cross-modality translation between MRI and PET imaging is challenging due to the distinct mechanisms underlying these modalities. Blood-based biomarkers (BBBMs) are revolutionizing Alzheimer's disease (AD) detection by identifying patients and quantifying brain amyloid levels. However, the potential of BBBMs to enhance PET image synthesis remains unexplored. In this paper, we performed a thorough study on the effect of incorporating BBBM into deep generative models. By evaluating three widely used cross-modality translation models, we found that BBBMs integration consistently enhances the generative quality across all models. By visual inspection of the generated results, we observed that PET images generated by CycleGAN exhibit the best visual fidelity. Based on these findings, we propose Plasma-CycleGAN, a novel generative model based on CycleGAN, to synthesize PET images from MRI using BBBMs as conditions. This is the first approach to integrate BBBMs in conditional cross-modality translation between MRI and PET.
Developing Univariate Neurodegeneration Biomarkers with Low-Rank and Sparse Subspace Decomposition
Oct 26, 2020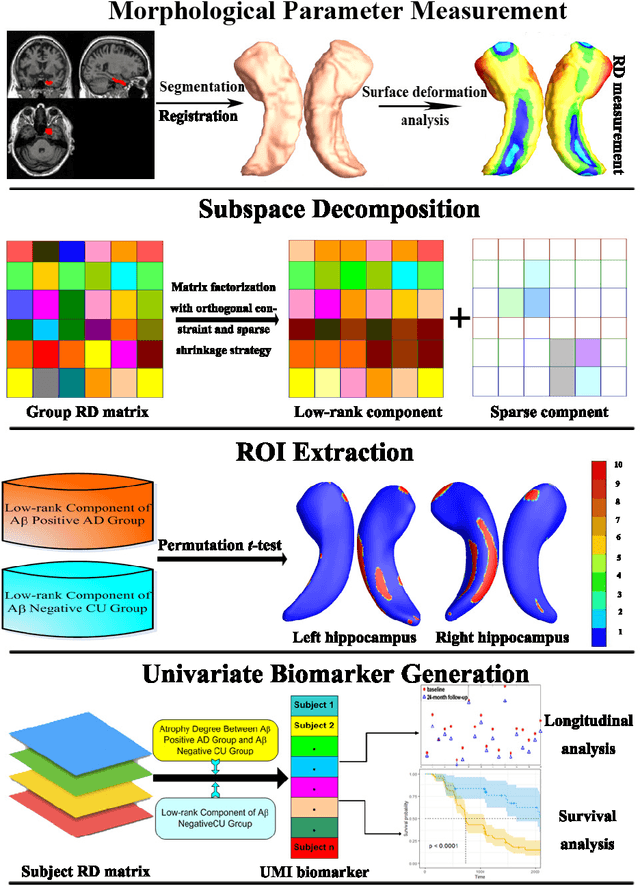
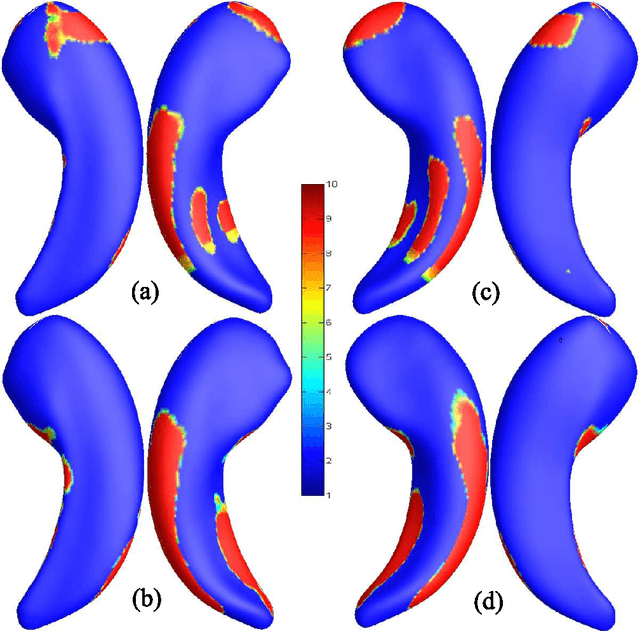
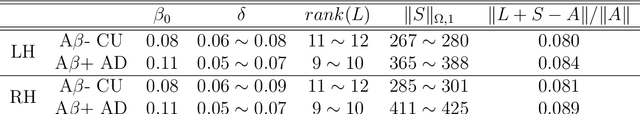
Abstract:Cognitive decline due to Alzheimer's disease (AD) is closely associated with brain structure alterations captured by structural magnetic resonance imaging (sMRI). It supports the validity to develop sMRI-based univariate neurodegeneration biomarkers (UNB). However, existing UNB work either fails to model large group variances or does not capture AD dementia (ADD) induced changes. We propose a novel low-rank and sparse subspace decomposition method capable of stably quantifying the morphological changes induced by ADD. Specifically, we propose a numerically efficient rank minimization mechanism to extract group common structure and impose regularization constraints to encode the original 3D morphometry connectivity. Further, we generate regions-of-interest (ROI) with group difference study between common subspaces of $A\beta+$ AD and $A\beta-$ cognitively unimpaired (CU) groups. A univariate morphometry index (UMI) is constructed from these ROIs by summarizing individual morphological characteristics weighted by normalized difference between $A\beta+$ AD and $A\beta-$ CU groups. We use hippocampal surface radial distance feature to compute the UMIs and validate our work in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. With hippocampal UMIs, the estimated minimum sample sizes needed to detect a 25$\%$ reduction in the mean annual change with 80$\%$ power and two-tailed $P=0.05$ are 116, 279 and 387 for the longitudinal $A\beta+$ AD, $A\beta+$ mild cognitive impairment (MCI) and $A\beta+$ CU groups, respectively. Additionally, for MCI patients, UMIs well correlate with hazard ratio of conversion to AD ($4.3$, $95\%$ CI=$2.3-8.2$) within 18 months. Our experimental results outperform traditional hippocampal volume measures and suggest the application of UMI as a potential UNB.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge