Yuanfeng Ji
nnMIL: A generalizable multiple instance learning framework for computational pathology
Nov 18, 2025Abstract:Computational pathology holds substantial promise for improving diagnosis and guiding treatment decisions. Recent pathology foundation models enable the extraction of rich patch-level representations from large-scale whole-slide images (WSIs), but current approaches for aggregating these features into slide-level predictions remain constrained by design limitations that hinder generalizability and reliability. Here, we developed nnMIL, a simple yet broadly applicable multiple-instance learning framework that connects patch-level foundation models to robust slide-level clinical inference. nnMIL introduces random sampling at both the patch and feature levels, enabling large-batch optimization, task-aware sampling strategies, and efficient and scalable training across datasets and model architectures. A lightweight aggregator performs sliding-window inference to generate ensemble slide-level predictions and supports principled uncertainty estimation. Across 40,000 WSIs encompassing 35 clinical tasks and four pathology foundation models, nnMIL consistently outperformed existing MIL methods for disease diagnosis, histologic subtyping, molecular biomarker detection, and pan- cancer prognosis prediction. It further demonstrated strong cross-model generalization, reliable uncertainty quantification, and robust survival stratification in multiple external cohorts. In conclusion, nnMIL offers a practical and generalizable solution for translating pathology foundation models into clinically meaningful predictions, advancing the development and deployment of reliable AI systems in real-world settings.
A Generative Foundation Model for Chest Radiography
Sep 04, 2025Abstract:The scarcity of well-annotated diverse medical images is a major hurdle for developing reliable AI models in healthcare. Substantial technical advances have been made in generative foundation models for natural images. Here we develop `ChexGen', a generative vision-language foundation model that introduces a unified framework for text-, mask-, and bounding box-guided synthesis of chest radiographs. Built upon the latent diffusion transformer architecture, ChexGen was pretrained on the largest curated chest X-ray dataset to date, consisting of 960,000 radiograph-report pairs. ChexGen achieves accurate synthesis of radiographs through expert evaluations and quantitative metrics. We demonstrate the utility of ChexGen for training data augmentation and supervised pretraining, which led to performance improvements across disease classification, detection, and segmentation tasks using a small fraction of training data. Further, our model enables the creation of diverse patient cohorts that enhance model fairness by detecting and mitigating demographic biases. Our study supports the transformative role of generative foundation models in building more accurate, data-efficient, and equitable medical AI systems.
A Survey of Scientific Large Language Models: From Data Foundations to Agent Frontiers
Aug 28, 2025



Abstract:Scientific Large Language Models (Sci-LLMs) are transforming how knowledge is represented, integrated, and applied in scientific research, yet their progress is shaped by the complex nature of scientific data. This survey presents a comprehensive, data-centric synthesis that reframes the development of Sci-LLMs as a co-evolution between models and their underlying data substrate. We formulate a unified taxonomy of scientific data and a hierarchical model of scientific knowledge, emphasizing the multimodal, cross-scale, and domain-specific challenges that differentiate scientific corpora from general natural language processing datasets. We systematically review recent Sci-LLMs, from general-purpose foundations to specialized models across diverse scientific disciplines, alongside an extensive analysis of over 270 pre-/post-training datasets, showing why Sci-LLMs pose distinct demands -- heterogeneous, multi-scale, uncertainty-laden corpora that require representations preserving domain invariance and enabling cross-modal reasoning. On evaluation, we examine over 190 benchmark datasets and trace a shift from static exams toward process- and discovery-oriented assessments with advanced evaluation protocols. These data-centric analyses highlight persistent issues in scientific data development and discuss emerging solutions involving semi-automated annotation pipelines and expert validation. Finally, we outline a paradigm shift toward closed-loop systems where autonomous agents based on Sci-LLMs actively experiment, validate, and contribute to a living, evolving knowledge base. Collectively, this work provides a roadmap for building trustworthy, continually evolving artificial intelligence (AI) systems that function as a true partner in accelerating scientific discovery.
MedITok: A Unified Tokenizer for Medical Image Synthesis and Interpretation
May 25, 2025Abstract:Advanced autoregressive models have reshaped multimodal AI. However, their transformative potential in medical imaging remains largely untapped due to the absence of a unified visual tokenizer -- one capable of capturing fine-grained visual structures for faithful image reconstruction and realistic image synthesis, as well as rich semantics for accurate diagnosis and image interpretation. To this end, we present MedITok, the first unified tokenizer tailored for medical images, encoding both low-level structural details and high-level clinical semantics within a unified latent space. To balance these competing objectives, we introduce a novel two-stage training framework: a visual representation alignment stage that cold-starts the tokenizer reconstruction learning with a visual semantic constraint, followed by a textual semantic representation alignment stage that infuses detailed clinical semantics into the latent space. Trained on the meticulously collected large-scale dataset with over 30 million medical images and 2 million image-caption pairs, MedITok achieves state-of-the-art performance on more than 30 datasets across 9 imaging modalities and 4 different tasks. By providing a unified token space for autoregressive modeling, MedITok supports a wide range of tasks in clinical diagnostics and generative healthcare applications. Model and code will be made publicly available at: https://github.com/Masaaki-75/meditok.
RetinaLogos: Fine-Grained Synthesis of High-Resolution Retinal Images Through Captions
May 19, 2025Abstract:The scarcity of high-quality, labelled retinal imaging data, which presents a significant challenge in the development of machine learning models for ophthalmology, hinders progress in the field. To synthesise Colour Fundus Photographs (CFPs), existing methods primarily relying on predefined disease labels face significant limitations. However, current methods remain limited, thus failing to generate images for broader categories with diverse and fine-grained anatomical structures. To overcome these challenges, we first introduce an innovative pipeline that creates a large-scale, synthetic Caption-CFP dataset comprising 1.4 million entries, called RetinaLogos-1400k. Specifically, RetinaLogos-1400k uses large language models (LLMs) to describe retinal conditions and key structures, such as optic disc configuration, vascular distribution, nerve fibre layers, and pathological features. Furthermore, based on this dataset, we employ a novel three-step training framework, called RetinaLogos, which enables fine-grained semantic control over retinal images and accurately captures different stages of disease progression, subtle anatomical variations, and specific lesion types. Extensive experiments demonstrate state-of-the-art performance across multiple datasets, with 62.07% of text-driven synthetic images indistinguishable from real ones by ophthalmologists. Moreover, the synthetic data improves accuracy by 10%-25% in diabetic retinopathy grading and glaucoma detection, thereby providing a scalable solution to augment ophthalmic datasets.
GMAI-VL-R1: Harnessing Reinforcement Learning for Multimodal Medical Reasoning
Apr 02, 2025Abstract:Recent advances in general medical AI have made significant strides, but existing models often lack the reasoning capabilities needed for complex medical decision-making. This paper presents GMAI-VL-R1, a multimodal medical reasoning model enhanced by reinforcement learning (RL) to improve its reasoning abilities. Through iterative training, GMAI-VL-R1 optimizes decision-making, significantly boosting diagnostic accuracy and clinical support. We also develop a reasoning data synthesis method, generating step-by-step reasoning data via rejection sampling, which further enhances the model's generalization. Experimental results show that after RL training, GMAI-VL-R1 excels in tasks such as medical image diagnosis and visual question answering. While the model demonstrates basic memorization with supervised fine-tuning, RL is crucial for true generalization. Our work establishes new evaluation benchmarks and paves the way for future advancements in medical reasoning models. Code, data, and model will be released at \href{https://github.com/uni-medical/GMAI-VL-R1}{this link}.
Towards Interpretable Counterfactual Generation via Multimodal Autoregression
Mar 29, 2025Abstract:Counterfactual medical image generation enables clinicians to explore clinical hypotheses, such as predicting disease progression, facilitating their decision-making. While existing methods can generate visually plausible images from disease progression prompts, they produce silent predictions that lack interpretation to verify how the generation reflects the hypothesized progression -- a critical gap for medical applications that require traceable reasoning. In this paper, we propose Interpretable Counterfactual Generation (ICG), a novel task requiring the joint generation of counterfactual images that reflect the clinical hypothesis and interpretation texts that outline the visual changes induced by the hypothesis. To enable ICG, we present ICG-CXR, the first dataset pairing longitudinal medical images with hypothetical progression prompts and textual interpretations. We further introduce ProgEmu, an autoregressive model that unifies the generation of counterfactual images and textual interpretations. We demonstrate the superiority of ProgEmu in generating progression-aligned counterfactuals and interpretations, showing significant potential in enhancing clinical decision support and medical education. Project page: https://progemu.github.io.
SegBook: A Simple Baseline and Cookbook for Volumetric Medical Image Segmentation
Nov 21, 2024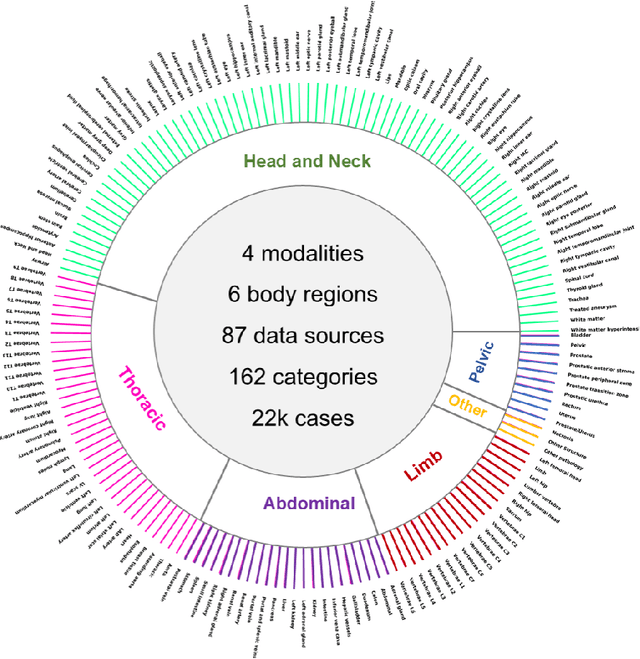
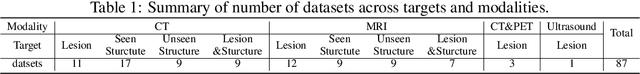
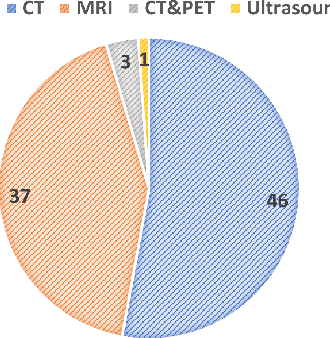
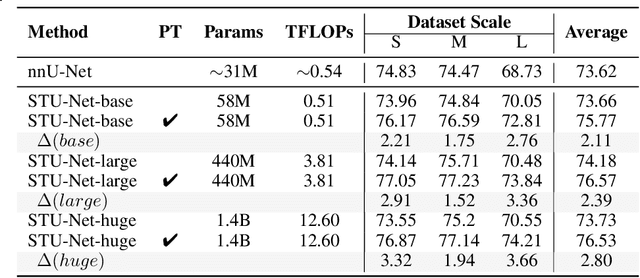
Abstract:Computed Tomography (CT) is one of the most popular modalities for medical imaging. By far, CT images have contributed to the largest publicly available datasets for volumetric medical segmentation tasks, covering full-body anatomical structures. Large amounts of full-body CT images provide the opportunity to pre-train powerful models, e.g., STU-Net pre-trained in a supervised fashion, to segment numerous anatomical structures. However, it remains unclear in which conditions these pre-trained models can be transferred to various downstream medical segmentation tasks, particularly segmenting the other modalities and diverse targets. To address this problem, a large-scale benchmark for comprehensive evaluation is crucial for finding these conditions. Thus, we collected 87 public datasets varying in modality, target, and sample size to evaluate the transfer ability of full-body CT pre-trained models. We then employed a representative model, STU-Net with multiple model scales, to conduct transfer learning across modalities and targets. Our experimental results show that (1) there may be a bottleneck effect concerning the dataset size in fine-tuning, with more improvement on both small- and large-scale datasets than medium-size ones. (2) Models pre-trained on full-body CT demonstrate effective modality transfer, adapting well to other modalities such as MRI. (3) Pre-training on the full-body CT not only supports strong performance in structure detection but also shows efficacy in lesion detection, showcasing adaptability across target tasks. We hope that this large-scale open evaluation of transfer learning can direct future research in volumetric medical image segmentation.
GMAI-VL & GMAI-VL-5.5M: A Large Vision-Language Model and A Comprehensive Multimodal Dataset Towards General Medical AI
Nov 21, 2024Abstract:Despite significant advancements in general artificial intelligence, such as GPT-4, their effectiveness in the medical domain (general medical AI, GMAI) remains constrained due to the absence of specialized medical knowledge. To address this challenge, we present GMAI-VL-5.5M, a comprehensive multimodal medical dataset created by converting hundreds of specialized medical datasets into meticulously constructed image-text pairs. This dataset features comprehensive task coverage, diverse modalities, and high-quality image-text data. Building upon this multimodal dataset, we propose GMAI-VL, a general medical vision-language model with a progressively three-stage training strategy. This approach significantly enhances the model's ability by integrating visual and textual information, thereby improving its ability to process multimodal data and support accurate diagnosis and clinical decision-making. Experimental evaluations demonstrate that GMAI-VL achieves state-of-the-art results across a wide range of multimodal medical tasks, such as visual question answering and medical image diagnosis. Our contributions include the development of the GMAI-VL-5.5M dataset, the introduction of the GMAI-VL model, and the establishment of new benchmarks in multiple medical domains. Code and dataset will be released at https://github.com/uni-medical/GMAI-VL.
Artificial Intelligence-Enhanced Couinaud Segmentation for Precision Liver Cancer Therapy
Nov 05, 2024



Abstract:Precision therapy for liver cancer necessitates accurately delineating liver sub-regions to protect healthy tissue while targeting tumors, which is essential for reducing recurrence and improving survival rates. However, the segmentation of hepatic segments, known as Couinaud segmentation, is challenging due to indistinct sub-region boundaries and the need for extensive annotated datasets. This study introduces LiverFormer, a novel Couinaud segmentation model that effectively integrates global context with low-level local features based on a 3D hybrid CNN-Transformer architecture. Additionally, a registration-based data augmentation strategy is equipped to enhance the segmentation performance with limited labeled data. Evaluated on CT images from 123 patients, LiverFormer demonstrated high accuracy and strong concordance with expert annotations across various metrics, allowing for enhanced treatment planning for surgery and radiation therapy. It has great potential to reduces complications and minimizes potential damages to surrounding tissue, leading to improved outcomes for patients undergoing complex liver cancer treatments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge