Junyi Gao
Augmenting Clinical Decision-Making with an Interactive and Interpretable AI Copilot: A Real-World User Study with Clinicians in Nephrology and Obstetrics
Jan 31, 2026Abstract:Clinician skepticism toward opaque AI hinders adoption in high-stakes healthcare. We present AICare, an interactive and interpretable AI copilot for collaborative clinical decision-making. By analyzing longitudinal electronic health records, AICare grounds dynamic risk predictions in scrutable visualizations and LLM-driven diagnostic recommendations. Through a within-subjects counterbalanced study with 16 clinicians across nephrology and obstetrics, we comprehensively evaluated AICare using objective measures (task completion time and error rate), subjective assessments (NASA-TLX, SUS, and confidence ratings), and semi-structured interviews. Our findings indicate AICare's reduced cognitive workload. Beyond performance metrics, qualitative analysis reveals that trust is actively constructed through verification, with interaction strategies diverging by expertise: junior clinicians used the system as cognitive scaffolding to structure their analysis, while experts engaged in adversarial verification to challenge the AI's logic. This work offers design implications for creating AI systems that function as transparent partners, accommodating diverse reasoning styles to augment rather than replace clinical judgment.
PyHealth 2.0: A Comprehensive Open-Source Toolkit for Accessible and Reproducible Clinical Deep Learning
Jan 23, 2026Abstract:Difficulty replicating baselines, high computational costs, and required domain expertise create persistent barriers to clinical AI research. To address these challenges, we introduce PyHealth 2.0, an enhanced clinical deep learning toolkit that enables predictive modeling in as few as 7 lines of code. PyHealth 2.0 offers three key contributions: (1) a comprehensive toolkit addressing reproducibility and compatibility challenges by unifying 15+ datasets, 20+ clinical tasks, 25+ models, 5+ interpretability methods, and uncertainty quantification including conformal prediction within a single framework that supports diverse clinical data modalities - signals, imaging, and electronic health records - with translation of 5+ medical coding standards; (2) accessibility-focused design accommodating multimodal data and diverse computational resources with up to 39x faster processing and 20x lower memory usage, enabling work from 16GB laptops to production systems; and (3) an active open-source community of 400+ members lowering domain expertise barriers through extensive documentation, reproducible research contributions, and collaborations with academic health systems and industry partners, including multi-language support via RHealth. PyHealth 2.0 establishes an open-source foundation and community advancing accessible, reproducible healthcare AI. Available at pip install pyhealth.
Magical: Medical Lay Language Generation via Semantic Invariance and Layperson-tailored Adaptation
Aug 12, 2025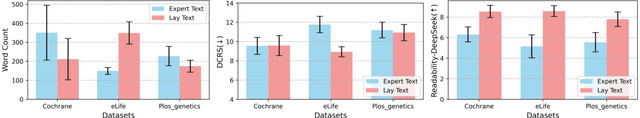
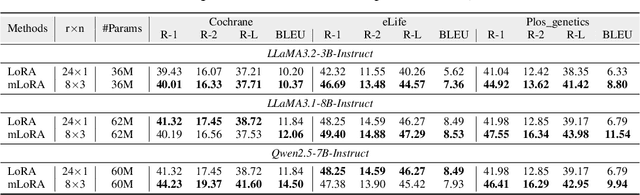
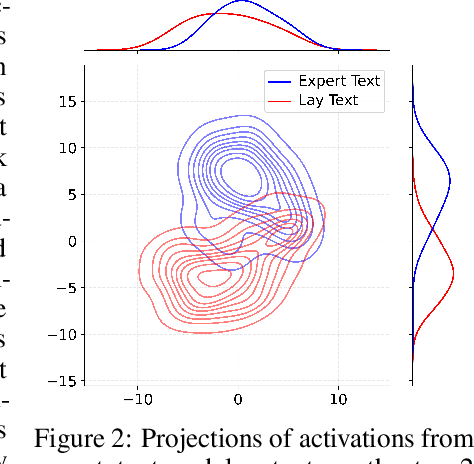
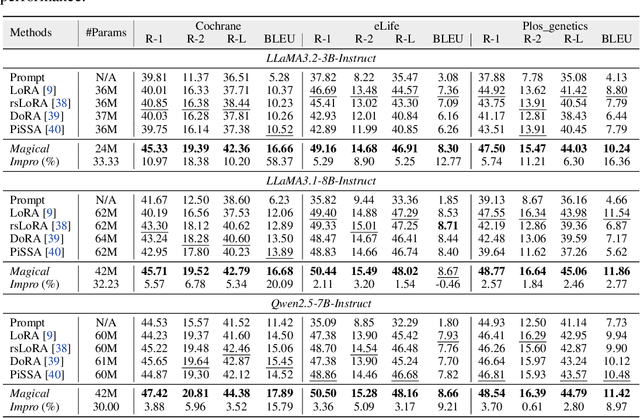
Abstract:Medical Lay Language Generation (MLLG) plays a vital role in improving the accessibility of complex scientific content for broader audiences. Recent literature to MLLG commonly employ parameter-efficient fine-tuning methods such as Low-Rank Adaptation (LoRA) to fine-tuning large language models (LLMs) using paired expert-lay language datasets. However, LoRA struggles with the challenges posed by multi-source heterogeneous MLLG datasets. Specifically, through a series of exploratory experiments, we reveal that standard LoRA fail to meet the requirement for semantic fidelity and diverse lay-style generation in MLLG task. To address these limitations, we propose Magical, an asymmetric LoRA architecture tailored for MLLG under heterogeneous data scenarios. Magical employs a shared matrix $A$ for abstractive summarization, along with multiple isolated matrices $B$ for diverse lay-style generation. To preserve semantic fidelity during the lay language generation process, Magical introduces a Semantic Invariance Constraint to mitigate semantic subspace shifts on matrix $A$. Furthermore, to better adapt to diverse lay-style generation, Magical incorporates the Recommendation-guided Switch, an externally interface to prompt the LLM to switch between different matrices $B$. Experimental results on three real-world lay language generation datasets demonstrate that Magical consistently outperforms prompt-based methods, vanilla LoRA, and its recent variants, while also reducing trainable parameters by 31.66%.
ConfAgents: A Conformal-Guided Multi-Agent Framework for Cost-Efficient Medical Diagnosis
Aug 06, 2025



Abstract:The efficacy of AI agents in healthcare research is hindered by their reliance on static, predefined strategies. This creates a critical limitation: agents can become better tool-users but cannot learn to become better strategic planners, a crucial skill for complex domains like healthcare. We introduce HealthFlow, a self-evolving AI agent that overcomes this limitation through a novel meta-level evolution mechanism. HealthFlow autonomously refines its own high-level problem-solving policies by distilling procedural successes and failures into a durable, strategic knowledge base. To anchor our research and facilitate reproducible evaluation, we introduce EHRFlowBench, a new benchmark featuring complex, realistic health data analysis tasks derived from peer-reviewed clinical research. Our comprehensive experiments demonstrate that HealthFlow's self-evolving approach significantly outperforms state-of-the-art agent frameworks. This work marks a necessary shift from building better tool-users to designing smarter, self-evolving task-managers, paving the way for more autonomous and effective AI for scientific discovery.
TrialPanorama: Database and Benchmark for Systematic Review and Design of Clinical Trials
May 22, 2025Abstract:Developing artificial intelligence (AI) for vertical domains requires a solid data foundation for both training and evaluation. In this work, we introduce TrialPanorama, a large-scale, structured database comprising 1,657,476 clinical trial records aggregated from 15 global sources. The database captures key aspects of trial design and execution, including trial setups, interventions, conditions, biomarkers, and outcomes, and links them to standard biomedical ontologies such as DrugBank and MedDRA. This structured and ontology-grounded design enables TrialPanorama to serve as a unified, extensible resource for a wide range of clinical trial tasks, including trial planning, design, and summarization. To demonstrate its utility, we derive a suite of benchmark tasks directly from the TrialPanorama database. The benchmark spans eight tasks across two categories: three for systematic review (study search, study screening, and evidence summarization) and five for trial design (arm design, eligibility criteria, endpoint selection, sample size estimation, and trial completion assessment). The experiments using five state-of-the-art large language models (LLMs) show that while general-purpose LLMs exhibit some zero-shot capability, their performance is still inadequate for high-stakes clinical trial workflows. We release TrialPanorama database and the benchmark to facilitate further research on AI for clinical trials.
MedAgentBoard: Benchmarking Multi-Agent Collaboration with Conventional Methods for Diverse Medical Tasks
May 18, 2025Abstract:The rapid advancement of Large Language Models (LLMs) has stimulated interest in multi-agent collaboration for addressing complex medical tasks. However, the practical advantages of multi-agent collaboration approaches remain insufficiently understood. Existing evaluations often lack generalizability, failing to cover diverse tasks reflective of real-world clinical practice, and frequently omit rigorous comparisons against both single-LLM-based and established conventional methods. To address this critical gap, we introduce MedAgentBoard, a comprehensive benchmark for the systematic evaluation of multi-agent collaboration, single-LLM, and conventional approaches. MedAgentBoard encompasses four diverse medical task categories: (1) medical (visual) question answering, (2) lay summary generation, (3) structured Electronic Health Record (EHR) predictive modeling, and (4) clinical workflow automation, across text, medical images, and structured EHR data. Our extensive experiments reveal a nuanced landscape: while multi-agent collaboration demonstrates benefits in specific scenarios, such as enhancing task completeness in clinical workflow automation, it does not consistently outperform advanced single LLMs (e.g., in textual medical QA) or, critically, specialized conventional methods that generally maintain better performance in tasks like medical VQA and EHR-based prediction. MedAgentBoard offers a vital resource and actionable insights, emphasizing the necessity of a task-specific, evidence-based approach to selecting and developing AI solutions in medicine. It underscores that the inherent complexity and overhead of multi-agent collaboration must be carefully weighed against tangible performance gains. All code, datasets, detailed prompts, and experimental results are open-sourced at https://medagentboard.netlify.app/.
InformGen: An AI Copilot for Accurate and Compliant Clinical Research Consent Document Generation
Apr 01, 2025Abstract:Leveraging large language models (LLMs) to generate high-stakes documents, such as informed consent forms (ICFs), remains a significant challenge due to the extreme need for regulatory compliance and factual accuracy. Here, we present InformGen, an LLM-driven copilot for accurate and compliant ICF drafting by optimized knowledge document parsing and content generation, with humans in the loop. We further construct a benchmark dataset comprising protocols and ICFs from 900 clinical trials. Experimental results demonstrate that InformGen achieves near 100% compliance with 18 core regulatory rules derived from FDA guidelines, outperforming a vanilla GPT-4o model by up to 30%. Additionally, a user study with five annotators shows that InformGen, when integrated with manual intervention, attains over 90% factual accuracy, significantly surpassing the vanilla GPT-4o model's 57%-82%. Crucially, InformGen ensures traceability by providing inline citations to source protocols, enabling easy verification and maintaining the highest standards of factual integrity.
ColaCare: Enhancing Electronic Health Record Modeling through Large Language Model-Driven Multi-Agent Collaboration
Oct 03, 2024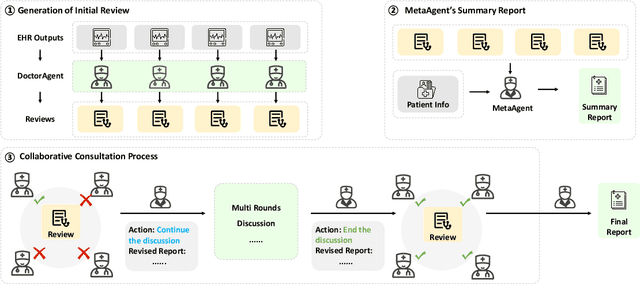
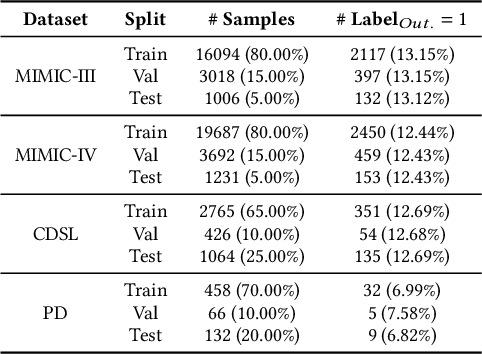
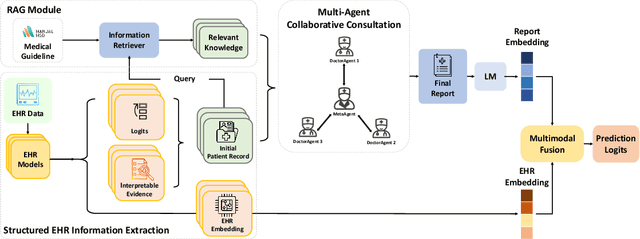
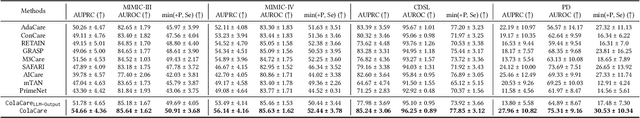
Abstract:We introduce ColaCare, a framework that enhances Electronic Health Record (EHR) modeling through multi-agent collaboration driven by Large Language Models (LLMs). Our approach seamlessly integrates domain-specific expert models with LLMs to bridge the gap between structured EHR data and text-based reasoning. Inspired by clinical consultations, ColaCare employs two types of agents: DoctorAgent and MetaAgent, which collaboratively analyze patient data. Expert models process and generate predictions from numerical EHR data, while LLM agents produce reasoning references and decision-making reports within the collaborative consultation framework. We additionally incorporate the Merck Manual of Diagnosis and Therapy (MSD) medical guideline within a retrieval-augmented generation (RAG) module for authoritative evidence support. Extensive experiments conducted on four distinct EHR datasets demonstrate ColaCare's superior performance in mortality prediction tasks, underscoring its potential to revolutionize clinical decision support systems and advance personalized precision medicine. The code, complete prompt templates, more case studies, etc. are publicly available at the anonymous link: https://colacare.netlify.app.
Is larger always better? Evaluating and prompting large language models for non-generative medical tasks
Jul 26, 2024Abstract:The use of Large Language Models (LLMs) in medicine is growing, but their ability to handle both structured Electronic Health Record (EHR) data and unstructured clinical notes is not well-studied. This study benchmarks various models, including GPT-based LLMs, BERT-based models, and traditional clinical predictive models, for non-generative medical tasks utilizing renowned datasets. We assessed 14 language models (9 GPT-based and 5 BERT-based) and 7 traditional predictive models using the MIMIC dataset (ICU patient records) and the TJH dataset (early COVID-19 EHR data), focusing on tasks such as mortality and readmission prediction, disease hierarchy reconstruction, and biomedical sentence matching, comparing both zero-shot and finetuned performance. Results indicated that LLMs exhibited robust zero-shot predictive capabilities on structured EHR data when using well-designed prompting strategies, frequently surpassing traditional models. However, for unstructured medical texts, LLMs did not outperform finetuned BERT models, which excelled in both supervised and unsupervised tasks. Consequently, while LLMs are effective for zero-shot learning on structured data, finetuned BERT models are more suitable for unstructured texts, underscoring the importance of selecting models based on specific task requirements and data characteristics to optimize the application of NLP technology in healthcare.
Adaptive Activation Steering: A Tuning-Free LLM Truthfulness Improvement Method for Diverse Hallucinations Categories
May 26, 2024Abstract:Recent studies have indicated that Large Language Models (LLMs) harbor an inherent understanding of truthfulness, yet often fail to express fully and generate false statements. This gap between "knowing" and "telling" poses a challenge for ensuring the truthfulness of generated content. To address this, we introduce Adaptive Activation Steering (ACT), a tuning-free method that adaptively shift LLM's activations in "truthful" direction during inference. ACT addresses diverse categories of hallucinations by utilizing diverse steering vectors and adjusting the steering intensity adaptively. Applied as an add-on across various models, ACT significantly improves truthfulness in LLaMA ($\uparrow$ 142\%), LLaMA2 ($\uparrow$ 24\%), Alpaca ($\uparrow$ 36\%), Vicuna ($\uparrow$ 28\%), and LLaMA2-Chat ($\uparrow$ 19\%). Furthermore, we verify ACT's scalability across larger models (13B, 33B, 65B), underscoring the adaptability of ACT to large-scale language models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge