Shivashankar Thati
InformGen: An AI Copilot for Accurate and Compliant Clinical Research Consent Document Generation
Apr 01, 2025Abstract:Leveraging large language models (LLMs) to generate high-stakes documents, such as informed consent forms (ICFs), remains a significant challenge due to the extreme need for regulatory compliance and factual accuracy. Here, we present InformGen, an LLM-driven copilot for accurate and compliant ICF drafting by optimized knowledge document parsing and content generation, with humans in the loop. We further construct a benchmark dataset comprising protocols and ICFs from 900 clinical trials. Experimental results demonstrate that InformGen achieves near 100% compliance with 18 core regulatory rules derived from FDA guidelines, outperforming a vanilla GPT-4o model by up to 30%. Additionally, a user study with five annotators shows that InformGen, when integrated with manual intervention, attains over 90% factual accuracy, significantly surpassing the vanilla GPT-4o model's 57%-82%. Crucially, InformGen ensures traceability by providing inline citations to source protocols, enabling easy verification and maintaining the highest standards of factual integrity.
Automatically Labeling $200B Life-Saving Datasets: A Large Clinical Trial Outcome Benchmark
Jun 13, 2024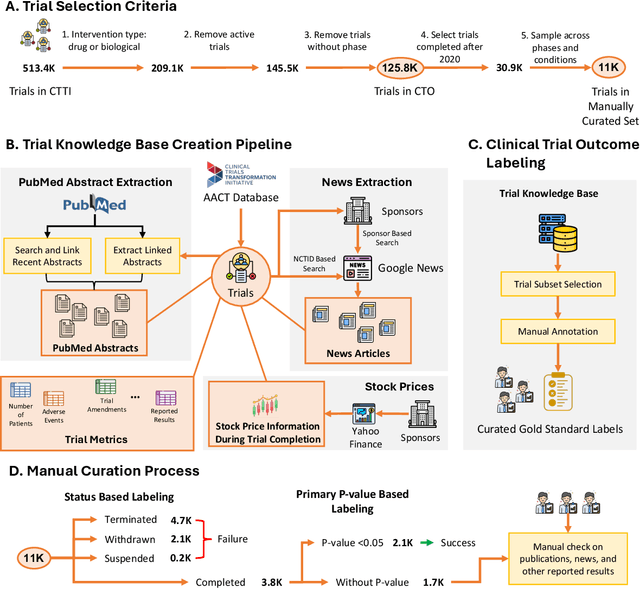
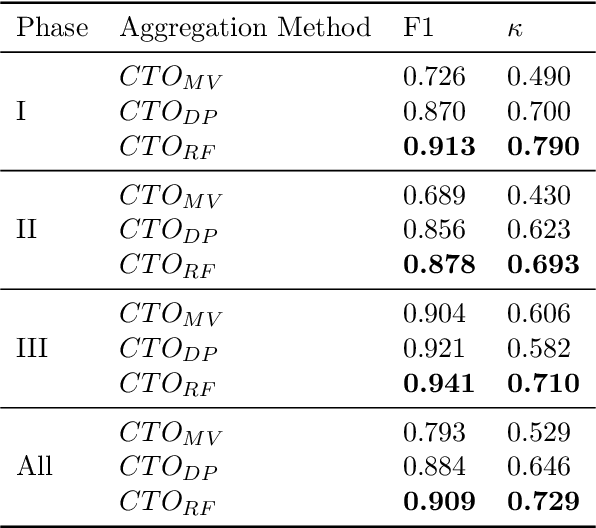
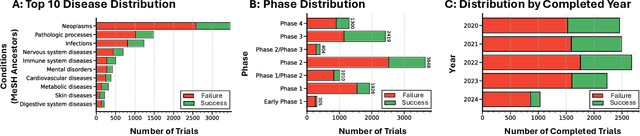
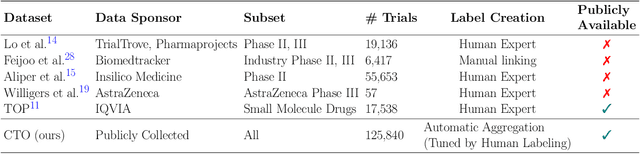
Abstract:The global cost of drug discovery and development exceeds $200 billion annually. The main results of drug discovery and development are the outcomes of clinical trials, which directly influence the regulatory approval of new drug candidates and ultimately affect patient outcomes. Despite their significance, large-scale, high-quality clinical trial outcome data are not readily available to the public. Suppose a large clinical trial outcome dataset is provided; machine learning researchers can potentially develop accurate prediction models using past trials and outcome labels, which could help prioritize and optimize therapeutic programs, ultimately benefiting patients. This paper introduces Clinical Trial Outcome (CTO) dataset, the largest trial outcome dataset with around 479K clinical trials, aggregating outcomes from multiple sources of weakly supervised labels, minimizing the noise from individual sources, and eliminating the need for human annotation. These sources include large language model (LLM) decisions on trial-related documents, news headline sentiments, stock prices of trial sponsors, trial linkages across phases, and other signals such as patient dropout rates and adverse events. CTO's labels show unprecedented agreement with supervised clinical trial outcome labels from test split of the supervised TOP dataset, with a 91 F1.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge