Junlin Hou
Free Lunch in Medical Image Foundation Model Pre-training via Randomized Synthesis and Disentanglement
Feb 12, 2026Abstract:Medical image foundation models (MIFMs) have demonstrated remarkable potential for a wide range of clinical tasks, yet their development is constrained by the scarcity, heterogeneity, and high cost of large-scale annotated datasets. Here, we propose RaSD (Randomized Synthesis and Disentanglement), a scalable framework for pre-training MIFMs entirely on synthetic data. By modeling anatomical structures and appearance variations with randomized Gaussian distributions, RaSD exposes models to sufficient multi-scale structural and appearance perturbations, forcing them to rely on invariant and task-relevant anatomical cues rather than dataset-specific textures, thereby enabling robust and transferable representation learning. We pre-trained RaSD on 1.2 million 3D volumes and 9.6 million 2D images, and extensively evaluated the resulting models across 6 imaging modalities, 48 datasets, and 56 downstream tasks. Across all evaluated downstream tasks, RaSD consistently outperforms training-from-scratch models, achieves the best performance on 17 tasks, and remains comparable to models pre-trained on large real datasets in most others. These results demonstrate that the capacity of synthetic data alone to drive robust representation learning. Our findings establish a paradigm shift in medical AI, demonstrating that synthetic data can serve as a "free lunch" for scalable, privacy-preserving, and clinically generalizable foundation models.
Benchmarking Real-World Medical Image Classification with Noisy Labels: Challenges, Practice, and Outlook
Dec 10, 2025Abstract:Learning from noisy labels remains a major challenge in medical image analysis, where annotation demands expert knowledge and substantial inter-observer variability often leads to inconsistent or erroneous labels. Despite extensive research on learning with noisy labels (LNL), the robustness of existing methods in medical imaging has not been systematically assessed. To address this gap, we introduce LNMBench, a comprehensive benchmark for Label Noise in Medical imaging. LNMBench encompasses \textbf{10} representative methods evaluated across 7 datasets, 6 imaging modalities, and 3 noise patterns, establishing a unified and reproducible framework for robustness evaluation under realistic conditions. Comprehensive experiments reveal that the performance of existing LNL methods degrades substantially under high and real-world noise, highlighting the persistent challenges of class imbalance and domain variability in medical data. Motivated by these findings, we further propose a simple yet effective improvement to enhance model robustness under such conditions. The LNMBench codebase is publicly released to facilitate standardized evaluation, promote reproducible research, and provide practical insights for developing noise-resilient algorithms in both research and real-world medical applications.The codebase is publicly available on https://github.com/myyy777/LNMBench.
A Versatile Pathology Co-pilot via Reasoning Enhanced Multimodal Large Language Model
Jul 23, 2025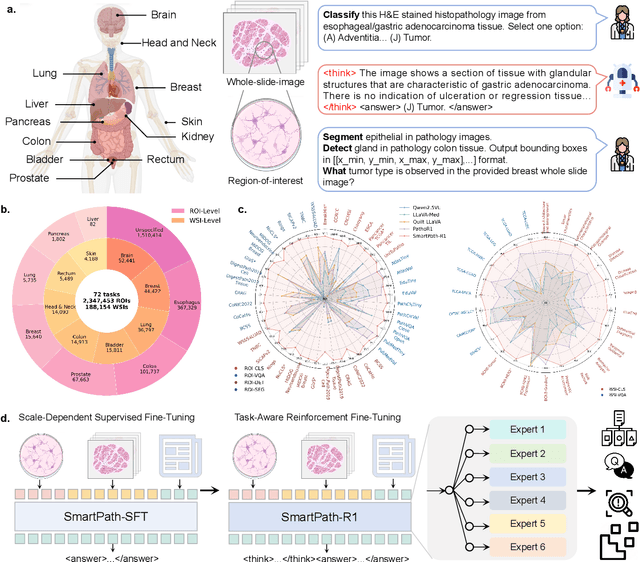
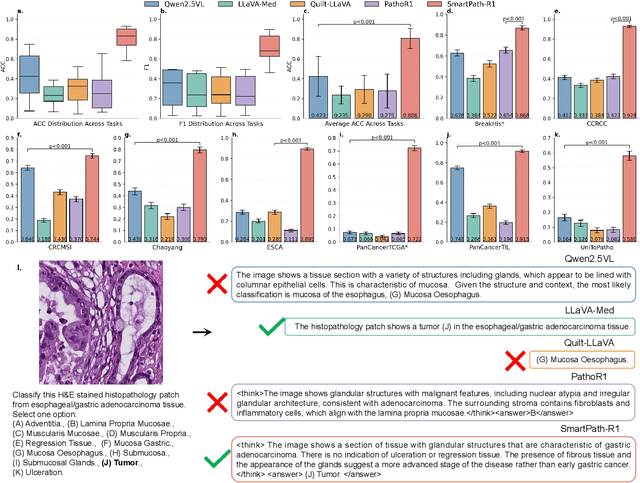
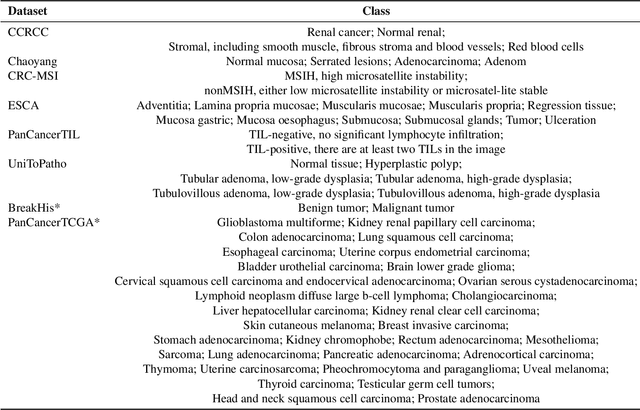
Abstract:Multimodal large language models (MLLMs) have emerged as powerful tools for computational pathology, offering unprecedented opportunities to integrate pathological images with language context for comprehensive diagnostic analysis. These models hold particular promise for automating complex tasks that traditionally require expert interpretation of pathologists. However, current MLLM approaches in pathology demonstrate significantly constrained reasoning capabilities, primarily due to their reliance on expensive chain-of-thought annotations. Additionally, existing methods remain limited to simplex application of visual question answering (VQA) at region-of-interest (ROI) level, failing to address the full spectrum of diagnostic needs such as ROI classification, detection, segmentation, whole-slide-image (WSI) classification and VQA in clinical practice. In this study, we present SmartPath-R1, a versatile MLLM capable of simultaneously addressing both ROI-level and WSI-level tasks while demonstrating robust pathological reasoning capability. Our framework combines scale-dependent supervised fine-tuning and task-aware reinforcement fine-tuning, which circumvents the requirement for chain-of-thought supervision by leveraging the intrinsic knowledge within MLLM. Furthermore, SmartPath-R1 integrates multiscale and multitask analysis through a mixture-of-experts mechanism, enabling dynamic processing for diverse tasks. We curate a large-scale dataset comprising 2.3M ROI samples and 188K WSI samples for training and evaluation. Extensive experiments across 72 tasks validate the effectiveness and superiority of the proposed approach. This work represents a significant step toward developing versatile, reasoning-enhanced AI systems for precision pathology.
Advancing Lung Disease Diagnosis in 3D CT Scans
Jul 01, 2025Abstract:To enable more accurate diagnosis of lung disease in chest CT scans, we propose a straightforward yet effective model. Firstly, we analyze the characteristics of 3D CT scans and remove non-lung regions, which helps the model focus on lesion-related areas and reduces computational cost. We adopt ResNeSt50 as a strong feature extractor, and use a weighted cross-entropy loss to mitigate class imbalance, especially for the underrepresented squamous cell carcinoma category. Our model achieves a Macro F1 Score of 0.80 on the validation set of the Fair Disease Diagnosis Challenge, demonstrating its strong performance in distinguishing between different lung conditions.
Segment Anything in Pathology Images with Natural Language
Jun 26, 2025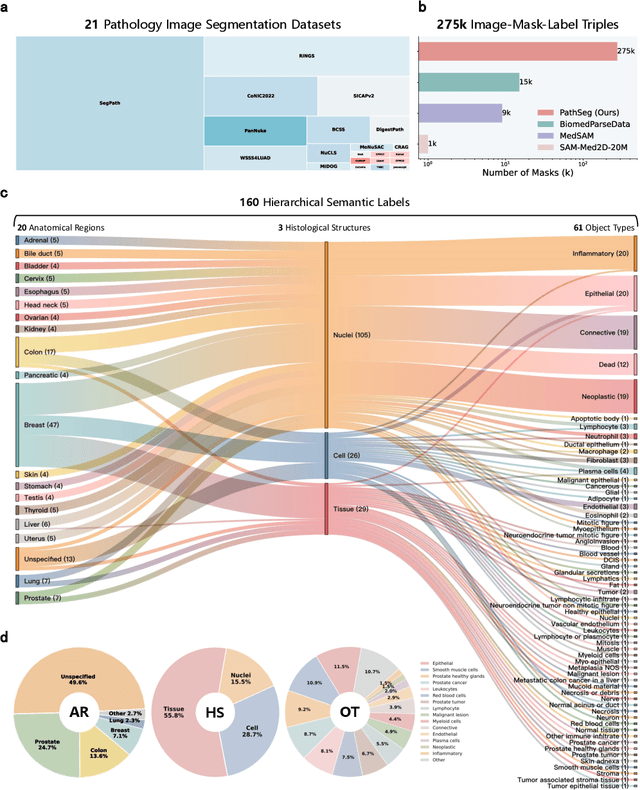
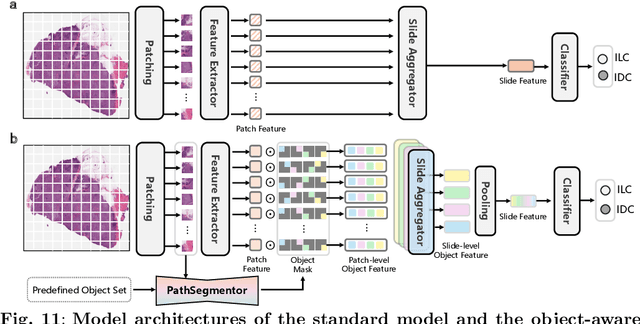
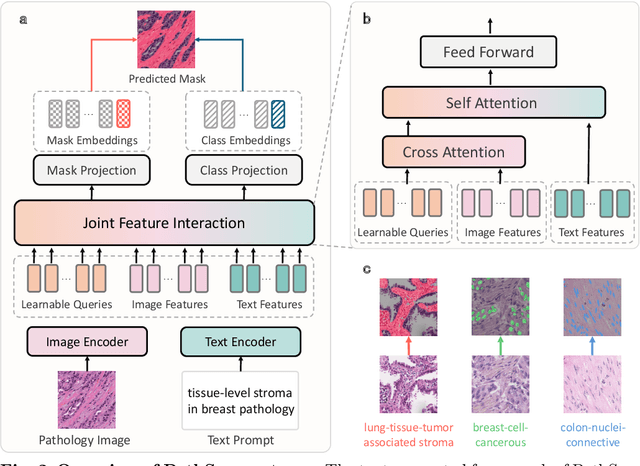
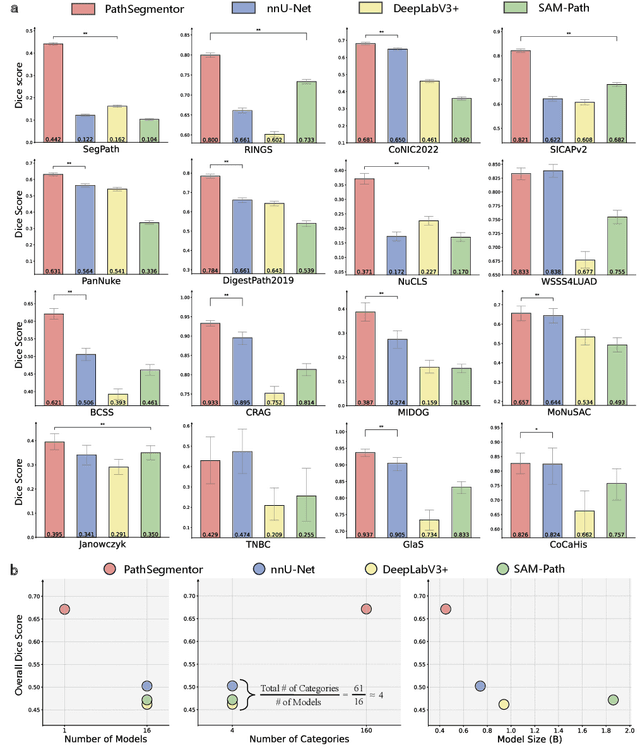
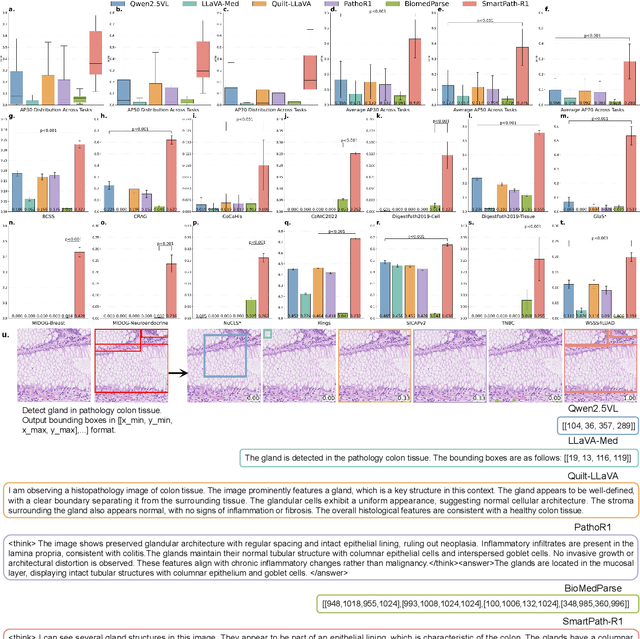
Abstract:Pathology image segmentation is crucial in computational pathology for analyzing histological features relevant to cancer diagnosis and prognosis. However, current methods face major challenges in clinical applications due to limited annotated data and restricted category definitions. To address these limitations, we propose PathSegmentor, the first text-prompted segmentation foundation model designed specifically for pathology images. We also introduce PathSeg , the largest and most comprehensive dataset for pathology segmentation, built from 17 public sources and containing 275k image-mask-label triples across 160 diverse categories. With PathSegmentor, users can perform semantic segmentation using natural language prompts, eliminating the need for laborious spatial inputs such as points or boxes. Extensive experiments demonstrate that PathSegmentor outperforms specialized models with higher accuracy and broader applicability, while maintaining a compact architecture. It significantly surpasses existing spatial- and text-prompted models by 0.145 and 0.429 in overall Dice scores, respectively, showing strong robustness in segmenting complex structures and generalizing to external datasets. Moreover, PathSegmentor's outputs enhance the interpretability of diagnostic models through feature importance estimation and imaging biomarker discovery, offering pathologists evidence-based support for clinical decision-making. This work advances the development of explainable AI in precision oncology.
EgoExo-Gen: Ego-centric Video Prediction by Watching Exo-centric Videos
Apr 16, 2025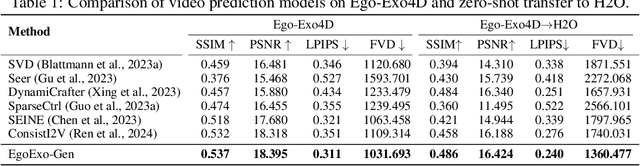
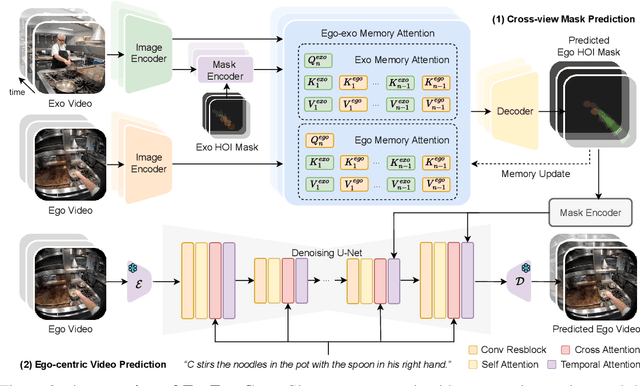
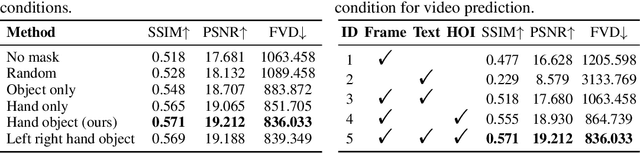
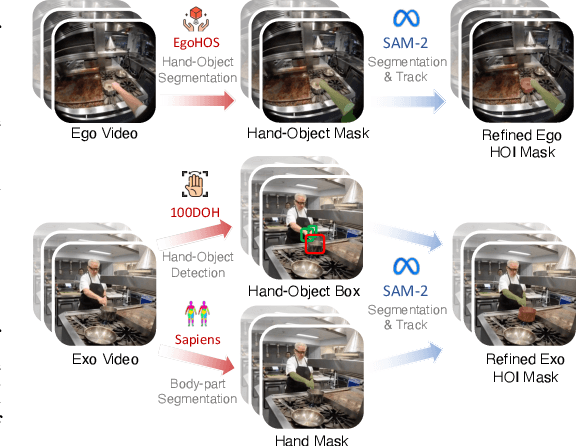
Abstract:Generating videos in the first-person perspective has broad application prospects in the field of augmented reality and embodied intelligence. In this work, we explore the cross-view video prediction task, where given an exo-centric video, the first frame of the corresponding ego-centric video, and textual instructions, the goal is to generate futur frames of the ego-centric video. Inspired by the notion that hand-object interactions (HOI) in ego-centric videos represent the primary intentions and actions of the current actor, we present EgoExo-Gen that explicitly models the hand-object dynamics for cross-view video prediction. EgoExo-Gen consists of two stages. First, we design a cross-view HOI mask prediction model that anticipates the HOI masks in future ego-frames by modeling the spatio-temporal ego-exo correspondence. Next, we employ a video diffusion model to predict future ego-frames using the first ego-frame and textual instructions, while incorporating the HOI masks as structural guidance to enhance prediction quality. To facilitate training, we develop an automated pipeline to generate pseudo HOI masks for both ego- and exo-videos by exploiting vision foundation models. Extensive experiments demonstrate that our proposed EgoExo-Gen achieves better prediction performance compared to previous video prediction models on the Ego-Exo4D and H2O benchmark datasets, with the HOI masks significantly improving the generation of hands and interactive objects in the ego-centric videos.
Cross-Fundus Transformer for Multi-modal Diabetic Retinopathy Grading with Cataract
Nov 01, 2024



Abstract:Diabetic retinopathy (DR) is a leading cause of blindness worldwide and a common complication of diabetes. As two different imaging tools for DR grading, color fundus photography (CFP) and infrared fundus photography (IFP) are highly-correlated and complementary in clinical applications. To the best of our knowledge, this is the first study that explores a novel multi-modal deep learning framework to fuse the information from CFP and IFP towards more accurate DR grading. Specifically, we construct a dual-stream architecture Cross-Fundus Transformer (CFT) to fuse the ViT-based features of two fundus image modalities. In particular, a meticulously engineered Cross-Fundus Attention (CFA) module is introduced to capture the correspondence between CFP and IFP images. Moreover, we adopt both the single-modality and multi-modality supervisions to maximize the overall performance for DR grading. Extensive experiments on a clinical dataset consisting of 1,713 pairs of multi-modal fundus images demonstrate the superiority of our proposed method. Our code will be released for public access.
Concept Complement Bottleneck Model for Interpretable Medical Image Diagnosis
Oct 20, 2024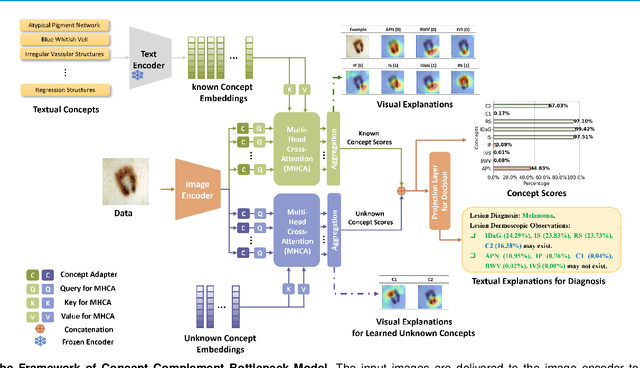
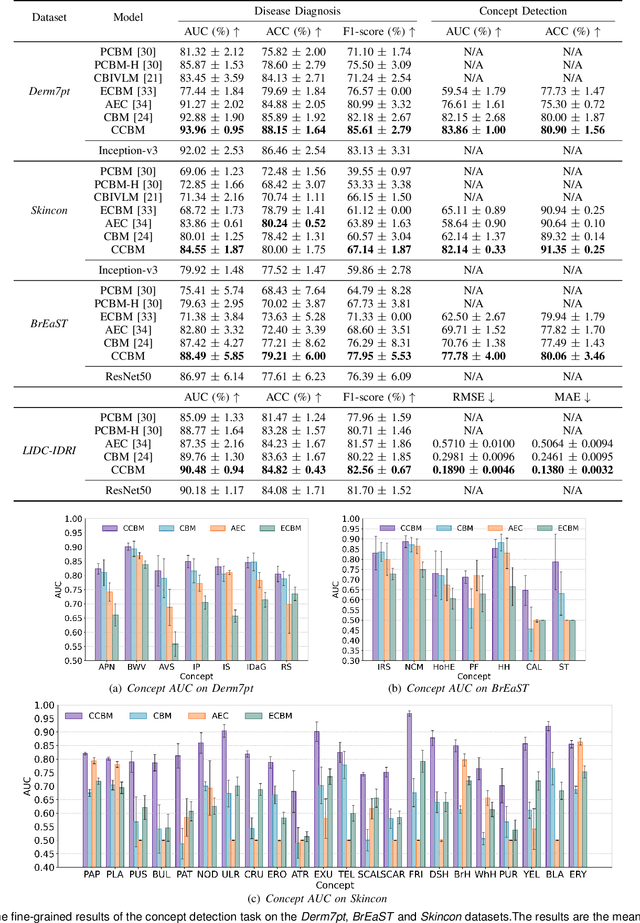
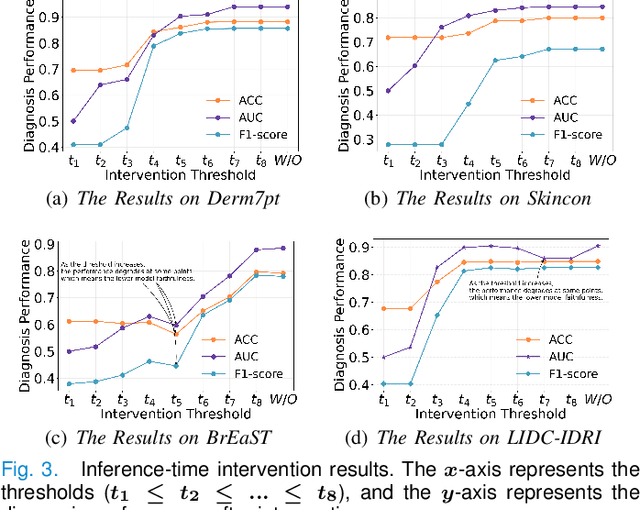
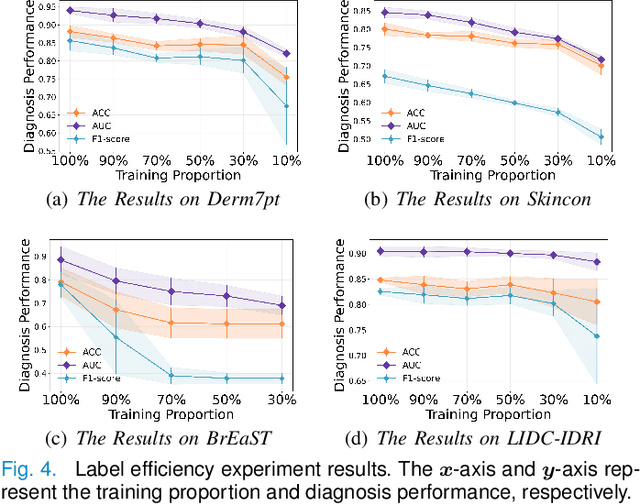
Abstract:Models based on human-understandable concepts have received extensive attention to improve model interpretability for trustworthy artificial intelligence in the field of medical image analysis. These methods can provide convincing explanations for model decisions but heavily rely on the detailed annotation of pre-defined concepts. Consequently, they may not be effective in cases where concepts or annotations are incomplete or low-quality. Although some methods automatically discover effective and new visual concepts rather than using pre-defined concepts or could find some human-understandable concepts via large Language models, they are prone to veering away from medical diagnostic evidence and are challenging to understand. In this paper, we propose a concept complement bottleneck model for interpretable medical image diagnosis with the aim of complementing the existing concept set and finding new concepts bridging the gap between explainable models. Specifically, we propose to use concept adapters for specific concepts to mine the concept differences and score concepts in their own attention channels to support almost fairly concept learning. Then, we devise a concept complement strategy to learn new concepts while jointly using known concepts to improve model performance. Comprehensive experiments on medical datasets demonstrate that our model outperforms the state-of-the-art competitors in concept detection and disease diagnosis tasks while providing diverse explanations to ensure model interpretability effectively.
Self-eXplainable AI for Medical Image Analysis: A Survey and New Outlooks
Oct 03, 2024



Abstract:The increasing demand for transparent and reliable models, particularly in high-stakes decision-making areas such as medical image analysis, has led to the emergence of eXplainable Artificial Intelligence (XAI). Post-hoc XAI techniques, which aim to explain black-box models after training, have been controversial in recent works concerning their fidelity to the models' predictions. In contrast, Self-eXplainable AI (S-XAI) offers a compelling alternative by incorporating explainability directly into the training process of deep learning models. This approach allows models to generate inherent explanations that are closely aligned with their internal decision-making processes. Such enhanced transparency significantly supports the trustworthiness, robustness, and accountability of AI systems in real-world medical applications. To facilitate the development of S-XAI methods for medical image analysis, this survey presents an comprehensive review across various image modalities and clinical applications. It covers more than 200 papers from three key perspectives: 1) input explainability through the integration of explainable feature engineering and knowledge graph, 2) model explainability via attention-based learning, concept-based learning, and prototype-based learning, and 3) output explainability by providing counterfactual explanation and textual explanation. Additionally, this paper outlines the desired characteristics of explainability and existing evaluation methods for assessing explanation quality. Finally, it discusses the major challenges and future research directions in developing S-XAI for medical image analysis.
MOSMOS: Multi-organ segmentation facilitated by medical report supervision
Sep 04, 2024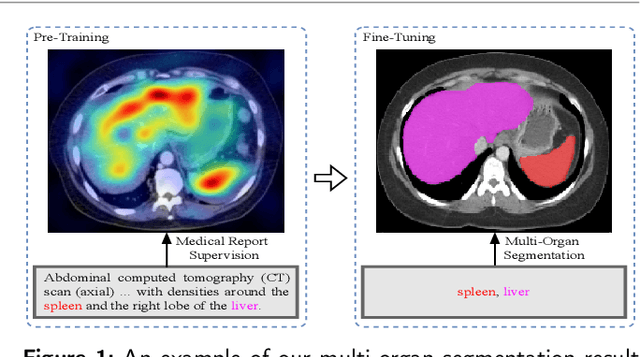
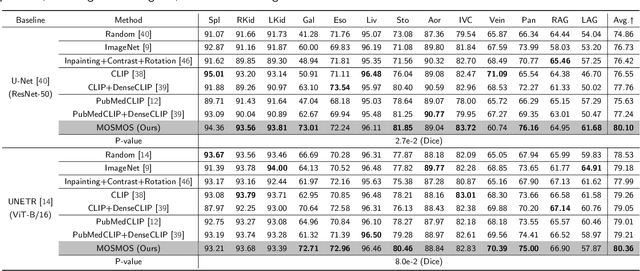
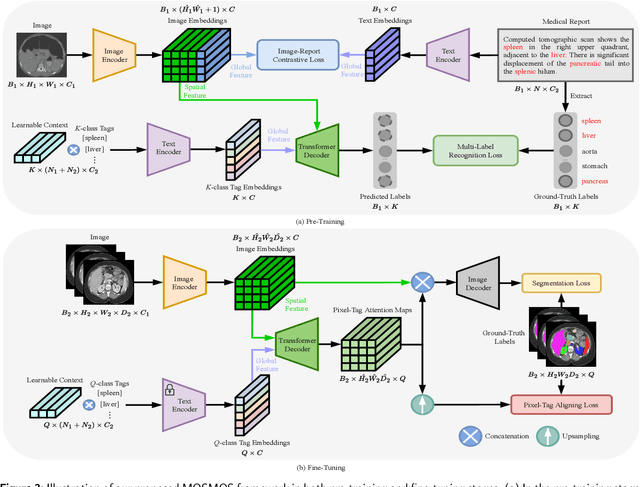
Abstract:Owing to a large amount of multi-modal data in modern medical systems, such as medical images and reports, Medical Vision-Language Pre-training (Med-VLP) has demonstrated incredible achievements in coarse-grained downstream tasks (i.e., medical classification, retrieval, and visual question answering). However, the problem of transferring knowledge learned from Med-VLP to fine-grained multi-organ segmentation tasks has barely been investigated. Multi-organ segmentation is challenging mainly due to the lack of large-scale fully annotated datasets and the wide variation in the shape and size of the same organ between individuals with different diseases. In this paper, we propose a novel pre-training & fine-tuning framework for Multi-Organ Segmentation by harnessing Medical repOrt Supervision (MOSMOS). Specifically, we first introduce global contrastive learning to maximally align the medical image-report pairs in the pre-training stage. To remedy the granularity discrepancy, we further leverage multi-label recognition to implicitly learn the semantic correspondence between image pixels and organ tags. More importantly, our pre-trained models can be transferred to any segmentation model by introducing the pixel-tag attention maps. Different network settings, i.e., 2D U-Net and 3D UNETR, are utilized to validate the generalization. We have extensively evaluated our approach using different diseases and modalities on BTCV, AMOS, MMWHS, and BRATS datasets. Experimental results in various settings demonstrate the effectiveness of our framework. This framework can serve as the foundation to facilitate future research on automatic annotation tasks under the supervision of medical reports.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge