Rui Feng
Georgia Institute of Technology
MAGA-Bench: Machine-Augment-Generated Text via Alignment Detection Benchmark
Jan 08, 2026Abstract:Large Language Models (LLMs) alignment is constantly evolving. Machine-Generated Text (MGT) is becoming increasingly difficult to distinguish from Human-Written Text (HWT). This has exacerbated abuse issues such as fake news and online fraud. Fine-tuned detectors' generalization ability is highly dependent on dataset quality, and simply expanding the sources of MGT is insufficient. Further augment of generation process is required. According to HC-Var's theory, enhancing the alignment of generated text can not only facilitate attacks on existing detectors to test their robustness, but also help improve the generalization ability of detectors fine-tuned on it. Therefore, we propose \textbf{M}achine-\textbf{A}ugment-\textbf{G}enerated Text via \textbf{A}lignment (MAGA). MAGA's pipeline achieves comprehensive alignment from prompt construction to reasoning process, among which \textbf{R}einforced \textbf{L}earning from \textbf{D}etectors \textbf{F}eedback (RLDF), systematically proposed by us, serves as a key component. In our experiments, the RoBERTa detector fine-tuned on MAGA training set achieved an average improvement of 4.60\% in generalization detection AUC. MAGA Dataset caused an average decrease of 8.13\% in the AUC of the selected detectors, expecting to provide indicative significance for future research on the generalization detection ability of detectors.
Genie Sim 3.0 : A High-Fidelity Comprehensive Simulation Platform for Humanoid Robot
Jan 05, 2026Abstract:The development of robust and generalizable robot learning models is critically contingent upon the availability of large-scale, diverse training data and reliable evaluation benchmarks. Collecting data in the physical world poses prohibitive costs and scalability challenges, and prevailing simulation benchmarks frequently suffer from fragmentation, narrow scope, or insufficient fidelity to enable effective sim-to-real transfer. To address these challenges, we introduce Genie Sim 3.0, a unified simulation platform for robotic manipulation. We present Genie Sim Generator, a large language model (LLM)-powered tool that constructs high-fidelity scenes from natural language instructions. Its principal strength resides in rapid and multi-dimensional generalization, facilitating the synthesis of diverse environments to support scalable data collection and robust policy evaluation. We introduce the first benchmark that pioneers the application of LLM for automated evaluation. It leverages LLM to mass-generate evaluation scenarios and employs Vision-Language Model (VLM) to establish an automated assessment pipeline. We also release an open-source dataset comprising more than 10,000 hours of synthetic data across over 200 tasks. Through systematic experimentation, we validate the robust zero-shot sim-to-real transfer capability of our open-source dataset, demonstrating that synthetic data can server as an effective substitute for real-world data under controlled conditions for scalable policy training. For code and dataset details, please refer to: https://github.com/AgibotTech/genie_sim.
EGGCodec: A Robust Neural Encodec Framework for EGG Reconstruction and F0 Extraction
Aug 12, 2025Abstract:This letter introduces EGGCodec, a robust neural Encodec framework engineered for electroglottography (EGG) signal reconstruction and F0 extraction. We propose a multi-scale frequency-domain loss function to capture the nuanced relationship between original and reconstructed EGG signals, complemented by a time-domain correlation loss to improve generalization and accuracy. Unlike conventional Encodec models that extract F0 directly from features, EGGCodec leverages reconstructed EGG signals, which more closely correspond to F0. By removing the conventional GAN discriminator, we streamline EGGCodec's training process without compromising efficiency, incurring only negligible performance degradation. Trained on a widely used EGG-inclusive dataset, extensive evaluations demonstrate that EGGCodec outperforms state-of-the-art F0 extraction schemes, reducing mean absolute error (MAE) from 14.14 Hz to 13.69 Hz, and improving voicing decision error (VDE) by 38.2\%. Moreover, extensive ablation experiments validate the contribution of each component of EGGCodec.
Exploring Gender Bias in Alzheimer's Disease Detection: Insights from Mandarin and Greek Speech Perception
Jul 16, 2025
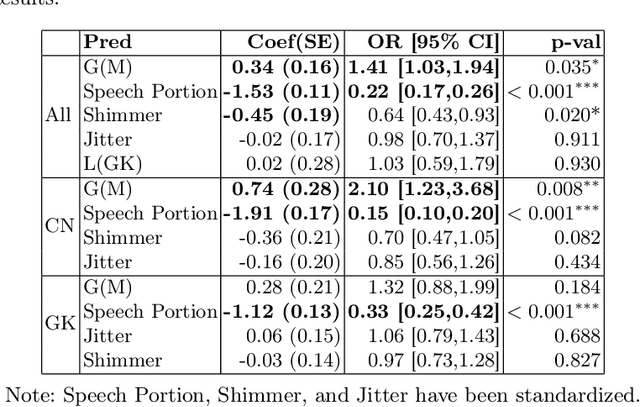
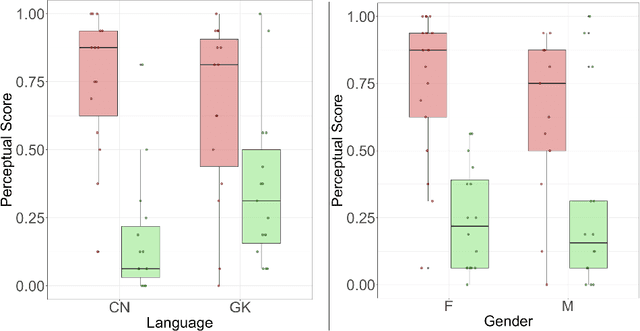
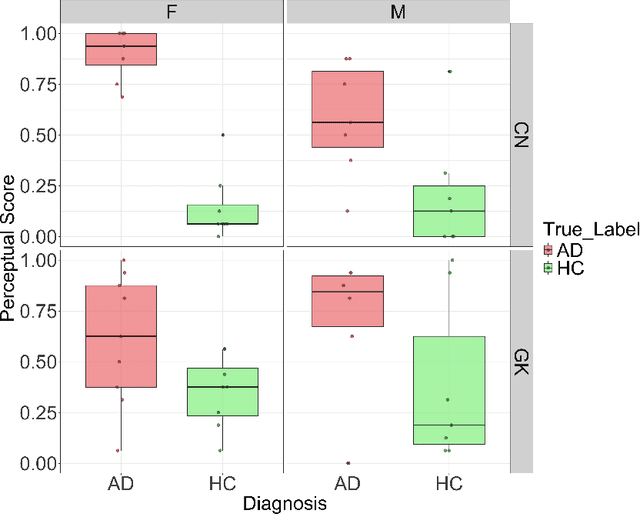
Abstract:Gender bias has been widely observed in speech perception tasks, influenced by the fundamental voicing differences between genders. This study reveals a gender bias in the perception of Alzheimer's Disease (AD) speech. In a perception experiment involving 16 Chinese listeners evaluating both Chinese and Greek speech, we identified that male speech was more frequently identified as AD, with this bias being particularly pronounced in Chinese speech. Acoustic analysis showed that shimmer values in male speech were significantly associated with AD perception, while speech portion exhibited a significant negative correlation with AD identification. Although language did not have a significant impact on AD perception, our findings underscore the critical role of gender bias in AD speech perception. This work highlights the necessity of addressing gender bias when developing AD detection models and calls for further research to validate model performance across different linguistic contexts.
Dual Semantic-Aware Network for Noise Suppressed Ultrasound Video Segmentation
Jul 10, 2025Abstract:Ultrasound imaging is a prevalent diagnostic tool known for its simplicity and non-invasiveness. However, its inherent characteristics often introduce substantial noise, posing considerable challenges for automated lesion or organ segmentation in ultrasound video sequences. To address these limitations, we propose the Dual Semantic-Aware Network (DSANet), a novel framework designed to enhance noise robustness in ultrasound video segmentation by fostering mutual semantic awareness between local and global features. Specifically, we introduce an Adjacent-Frame Semantic-Aware (AFSA) module, which constructs a channel-wise similarity matrix to guide feature fusion across adjacent frames, effectively mitigating the impact of random noise without relying on pixel-level relationships. Additionally, we propose a Local-and-Global Semantic-Aware (LGSA) module that reorganizes and fuses temporal unconditional local features, which capture spatial details independently at each frame, with conditional global features that incorporate temporal context from adjacent frames. This integration facilitates multi-level semantic representation, significantly improving the model's resilience to noise interference. Extensive evaluations on four benchmark datasets demonstrate that DSANet substantially outperforms state-of-the-art methods in segmentation accuracy. Moreover, since our model avoids pixel-level feature dependencies, it achieves significantly higher inference FPS than video-based methods, and even surpasses some image-based models. Code can be found in \href{https://github.com/ZhouL2001/DSANet}{DSANet}
Advancing Lung Disease Diagnosis in 3D CT Scans
Jul 01, 2025Abstract:To enable more accurate diagnosis of lung disease in chest CT scans, we propose a straightforward yet effective model. Firstly, we analyze the characteristics of 3D CT scans and remove non-lung regions, which helps the model focus on lesion-related areas and reduces computational cost. We adopt ResNeSt50 as a strong feature extractor, and use a weighted cross-entropy loss to mitigate class imbalance, especially for the underrepresented squamous cell carcinoma category. Our model achieves a Macro F1 Score of 0.80 on the validation set of the Fair Disease Diagnosis Challenge, demonstrating its strong performance in distinguishing between different lung conditions.
Beyond Manual Transcripts: The Potential of Automated Speech Recognition Errors in Improving Alzheimer's Disease Detection
May 26, 2025Abstract:Recent breakthroughs in Automatic Speech Recognition (ASR) have enabled fully automated Alzheimer's Disease (AD) detection using ASR transcripts. Nonetheless, the impact of ASR errors on AD detection remains poorly understood. This paper fills the gap. We conduct a comprehensive study on AD detection using transcripts from various ASR models and their synthesized speech on the ADReSS dataset. Experimental results reveal that certain ASR transcripts (ASR-synthesized speech) outperform manual transcripts (manual-synthesized speech) in detection accuracy, suggesting that ASR errors may provide valuable cues for improving AD detection. Additionally, we propose a cross-attention-based interpretability model that not only identifies these cues but also achieves superior or comparable performance to the baseline. Furthermore, we utilize this model to unveil AD-related patterns within pre-trained embeddings. Our study offers novel insights into the potential of ASR models for AD detection.
Decoding Speaker-Normalized Pitch from EEG for Mandarin Perception
May 26, 2025Abstract:The same speech content produced by different speakers exhibits significant differences in pitch contour, yet listeners' semantic perception remains unaffected. This phenomenon may stem from the brain's perception of pitch contours being independent of individual speakers' pitch ranges. In this work, we recorded electroencephalogram (EEG) while participants listened to Mandarin monosyllables with varying tones, phonemes, and speakers. The CE-ViViT model is proposed to decode raw or speaker-normalized pitch contours directly from EEG. Experimental results demonstrate that the proposed model can decode pitch contours with modest errors, achieving performance comparable to state-of-the-art EEG regression methods. Moreover, speaker-normalized pitch contours were decoded more accurately, supporting the neural encoding of relative pitch.
Leveraging Cascaded Binary Classification and Multimodal Fusion for Dementia Detection through Spontaneous Speech
May 26, 2025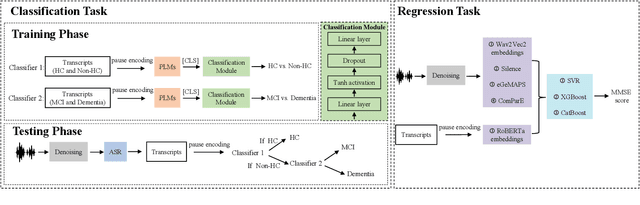
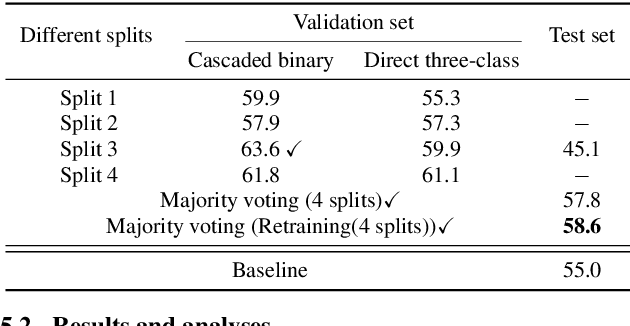
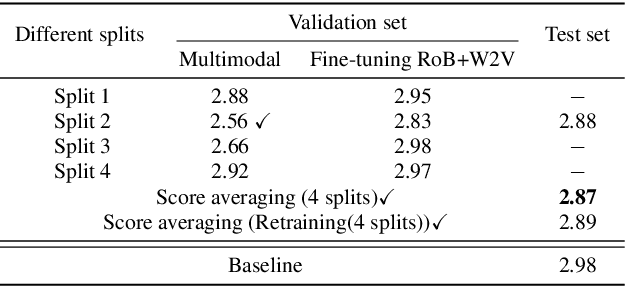
Abstract:This paper presents our submission to the PROCESS Challenge 2025, focusing on spontaneous speech analysis for early dementia detection. For the three-class classification task (Healthy Control, Mild Cognitive Impairment, and Dementia), we propose a cascaded binary classification framework that fine-tunes pre-trained language models and incorporates pause encoding to better capture disfluencies. This design streamlines multi-class classification and addresses class imbalance by restructuring the decision process. For the Mini-Mental State Examination score regression task, we develop an enhanced multimodal fusion system that combines diverse acoustic and linguistic features. Separate regression models are trained on individual feature sets, with ensemble learning applied through score averaging. Experimental results on the test set outperform the baselines provided by the organizers in both tasks, demonstrating the robustness and effectiveness of our approach.
AOR: Anatomical Ontology-Guided Reasoning for Medical Large Multimodal Model in Chest X-Ray Interpretation
May 05, 2025Abstract:Chest X-rays (CXRs) are the most frequently performed imaging examinations in clinical settings. Recent advancements in Large Multimodal Models (LMMs) have enabled automated CXR interpretation, enhancing diagnostic accuracy and efficiency. However, despite their strong visual understanding, current Medical LMMs (MLMMs) still face two major challenges: (1) Insufficient region-level understanding and interaction, and (2) Limited accuracy and interpretability due to single-step reasoning. In this paper, we empower MLMMs with anatomy-centric reasoning capabilities to enhance their interactivity and explainability. Specifically, we first propose an Anatomical Ontology-Guided Reasoning (AOR) framework, which centers on cross-modal region-level information to facilitate multi-step reasoning. Next, under the guidance of expert physicians, we develop AOR-Instruction, a large instruction dataset for MLMMs training. Our experiments demonstrate AOR's superior performance in both VQA and report generation tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge