Hyewon Jeong
Tiered Agentic Oversight: A Hierarchical Multi-Agent System for AI Safety in Healthcare
Jun 14, 2025Abstract:Current large language models (LLMs), despite their power, can introduce safety risks in clinical settings due to limitations such as poor error detection and single point of failure. To address this, we propose Tiered Agentic Oversight (TAO), a hierarchical multi-agent framework that enhances AI safety through layered, automated supervision. Inspired by clinical hierarchies (e.g., nurse, physician, specialist), TAO conducts agent routing based on task complexity and agent roles. Leveraging automated inter- and intra-tier collaboration and role-playing, TAO creates a robust safety framework. Ablation studies reveal that TAO's superior performance is driven by its adaptive tiered architecture, which improves safety by over 3.2% compared to static single-tier configurations; the critical role of its lower tiers, particularly tier 1, whose removal most significantly impacts safety; and the strategic assignment of more advanced LLM to these initial tiers, which boosts performance by over 2% compared to less optimal allocations while achieving near-peak safety efficiently. These mechanisms enable TAO to outperform single-agent and multi-agent frameworks in 4 out of 5 healthcare safety benchmarks, showing up to an 8.2% improvement over the next-best methods in these evaluations. Finally, we validate TAO via an auxiliary clinician-in-the-loop study where integrating expert feedback improved TAO's accuracy in medical triage from 40% to 60%.
MedPAIR: Measuring Physicians and AI Relevance Alignment in Medical Question Answering
May 29, 2025Abstract:Large Language Models (LLMs) have demonstrated remarkable performance on various medical question-answering (QA) benchmarks, including standardized medical exams. However, correct answers alone do not ensure correct logic, and models may reach accurate conclusions through flawed processes. In this study, we introduce the MedPAIR (Medical Dataset Comparing Physicians and AI Relevance Estimation and Question Answering) dataset to evaluate how physician trainees and LLMs prioritize relevant information when answering QA questions. We obtain annotations on 1,300 QA pairs from 36 physician trainees, labeling each sentence within the question components for relevance. We compare these relevance estimates to those for LLMs, and further evaluate the impact of these "relevant" subsets on downstream task performance for both physician trainees and LLMs. We find that LLMs are frequently not aligned with the content relevance estimates of physician trainees. After filtering out physician trainee-labeled irrelevant sentences, accuracy improves for both the trainees and the LLMs. All LLM and physician trainee-labeled data are available at: http://medpair.csail.mit.edu/.
BehaviorSFT: Behavioral Token Conditioning for Clinical Agents Across the Proactivity Spectrum
May 27, 2025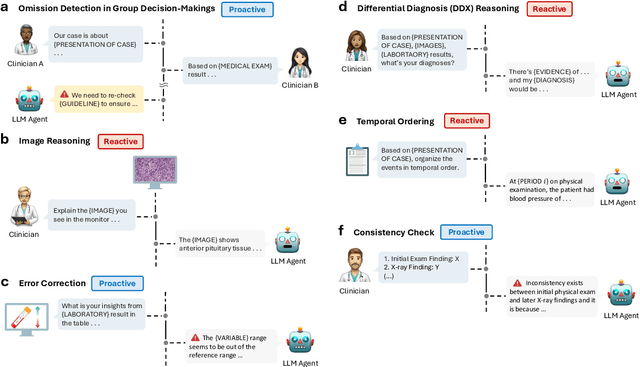
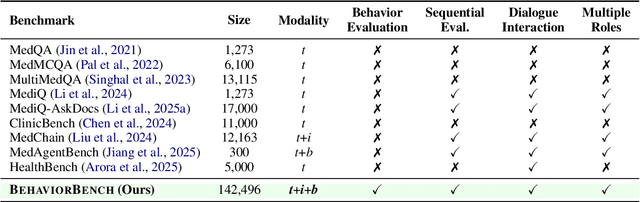
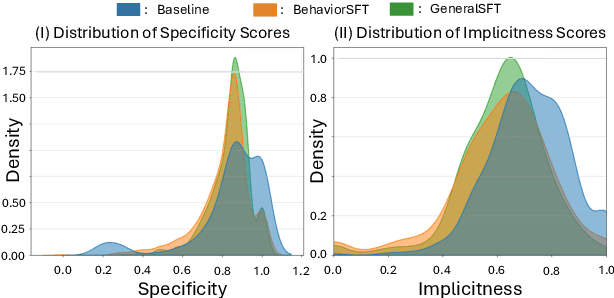
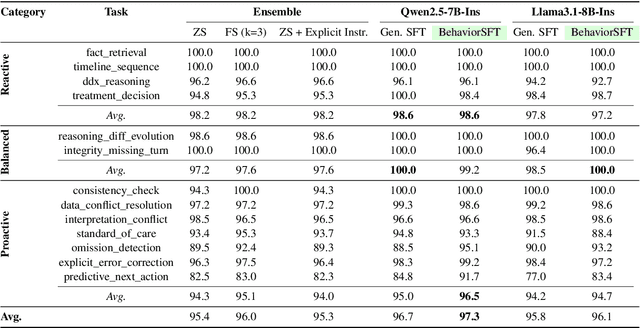
Abstract:Large Language Models (LLMs) as clinical agents require careful behavioral adaptation. While adept at reactive tasks (e.g., diagnosis reasoning), LLMs often struggle with proactive engagement, like unprompted identification of critical missing information or risks. We introduce BehaviorBench, a comprehensive dataset to evaluate agent behaviors across a clinical assistance spectrum, ranging from reactive query responses to proactive interventions (e.g., clarifying ambiguities, flagging overlooked critical data). Our BehaviorBench experiments reveal LLMs' inconsistent proactivity. To address this, we propose BehaviorSFT, a novel training strategy using behavioral tokens to explicitly condition LLMs for dynamic behavioral selection along this spectrum. BehaviorSFT boosts performance, achieving up to 97.3% overall Macro F1 on BehaviorBench and improving proactive task scores (e.g., from 95.0% to 96.5% for Qwen2.5-7B-Ins). Crucially, blind clinician evaluations confirmed BehaviorSFT-trained agents exhibit more realistic clinical behavior, striking a superior balance between helpful proactivity (e.g., timely, relevant suggestions) and necessary restraint (e.g., avoiding over-intervention) versus standard fine-tuning or explicit instructed agents.
RelCon: Relative Contrastive Learning for a Motion Foundation Model for Wearable Data
Nov 27, 2024



Abstract:We present RelCon, a novel self-supervised \textit{Rel}ative \textit{Con}trastive learning approach that uses a learnable distance measure in combination with a softened contrastive loss for training an motion foundation model from wearable sensors. The learnable distance measure captures motif similarity and domain-specific semantic information such as rotation invariance. The learned distance provides a measurement of semantic similarity between a pair of accelerometer time-series segments, which is used to measure the distance between an anchor and various other sampled candidate segments. The self-supervised model is trained on 1 billion segments from 87,376 participants from a large wearables dataset. The model achieves strong performance across multiple downstream tasks, encompassing both classification and regression. To our knowledge, we are the first to show the generalizability of a self-supervised learning model with motion data from wearables across distinct evaluation tasks.
Identifying Differential Patient Care Through Inverse Intent Inference
Nov 11, 2024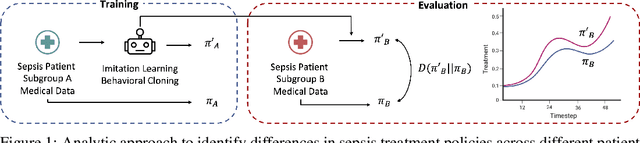

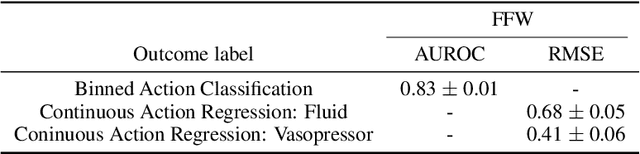
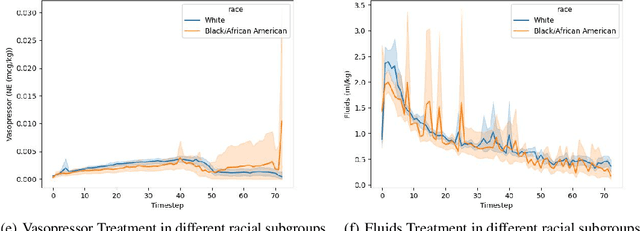
Abstract:Sepsis is a life-threatening condition defined by end-organ dysfunction due to a dysregulated host response to infection. Although the Surviving Sepsis Campaign has launched and has been releasing sepsis treatment guidelines to unify and normalize the care for sepsis patients, it has been reported in numerous studies that disparities in care exist across the trajectory of patient stay in the emergency department and intensive care unit. Here, we apply a number of reinforcement learning techniques including behavioral cloning, imitation learning, and inverse reinforcement learning, to learn the optimal policy in the management of septic patient subgroups using expert demonstrations. Then we estimate the counterfactual optimal policies by applying the model to another subset of unseen medical populations and identify the difference in cure by comparing it to the real policy. Our data comes from the sepsis cohort of MIMIC-IV and the clinical data warehouses of the Mass General Brigham healthcare system. The ultimate objective of this work is to use the optimal learned policy function to estimate the counterfactual treatment policy and identify deviations across sub-populations of interest. We hope this approach would help us identify any disparities in care and also changes in cure in response to the publication of national sepsis treatment guidelines.
MEDS-Tab: Automated tabularization and baseline methods for MEDS datasets
Oct 31, 2024



Abstract:Effective, reliable, and scalable development of machine learning (ML) solutions for structured electronic health record (EHR) data requires the ability to reliably generate high-quality baseline models for diverse supervised learning tasks in an efficient and performant manner. Historically, producing such baseline models has been a largely manual effort--individual researchers would need to decide on the particular featurization and tabularization processes to apply to their individual raw, longitudinal data; and then train a supervised model over those data to produce a baseline result to compare novel methods against, all for just one task and one dataset. In this work, powered by complementary advances in core data standardization through the MEDS framework, we dramatically simplify and accelerate this process of tabularizing irregularly sampled time-series data, providing researchers the ability to automatically and scalably featurize and tabularize their longitudinal EHR data across tens of thousands of individual features, hundreds of millions of clinical events, and diverse windowing horizons and aggregation strategies, all before ultimately leveraging these tabular data to automatically produce high-caliber XGBoost baselines in a highly computationally efficient manner. This system scales to dramatically larger datasets than tabularization tools currently available to the community and enables researchers with any MEDS format dataset to immediately begin producing reliable and performant baseline prediction results on various tasks, with minimal human effort required. This system will greatly enhance the reliability, reproducibility, and ease of development of powerful ML solutions for health problems across diverse datasets and clinical settings.
A Demonstration of Adaptive Collaboration of Large Language Models for Medical Decision-Making
Oct 31, 2024



Abstract:Medical Decision-Making (MDM) is a multi-faceted process that requires clinicians to assess complex multi-modal patient data patient, often collaboratively. Large Language Models (LLMs) promise to streamline this process by synthesizing vast medical knowledge and multi-modal health data. However, single-agent are often ill-suited for nuanced medical contexts requiring adaptable, collaborative problem-solving. Our MDAgents addresses this need by dynamically assigning collaboration structures to LLMs based on task complexity, mimicking real-world clinical collaboration and decision-making. This framework improves diagnostic accuracy and supports adaptive responses in complex, real-world medical scenarios, making it a valuable tool for clinicians in various healthcare settings, and at the same time, being more efficient in terms of computing cost than static multi-agent decision making methods.
Adaptive Collaboration Strategy for LLMs in Medical Decision Making
Apr 22, 2024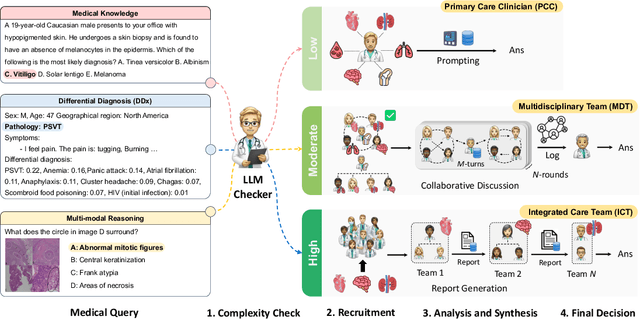
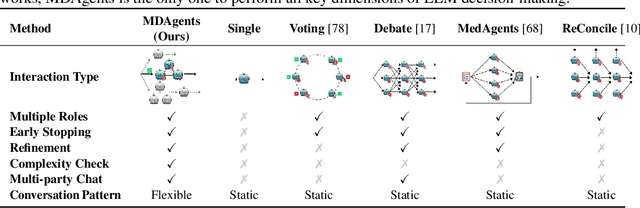
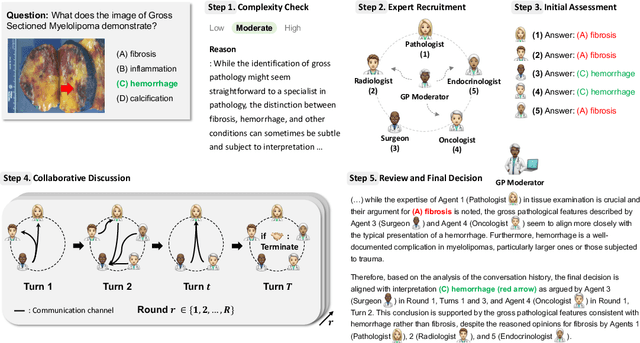
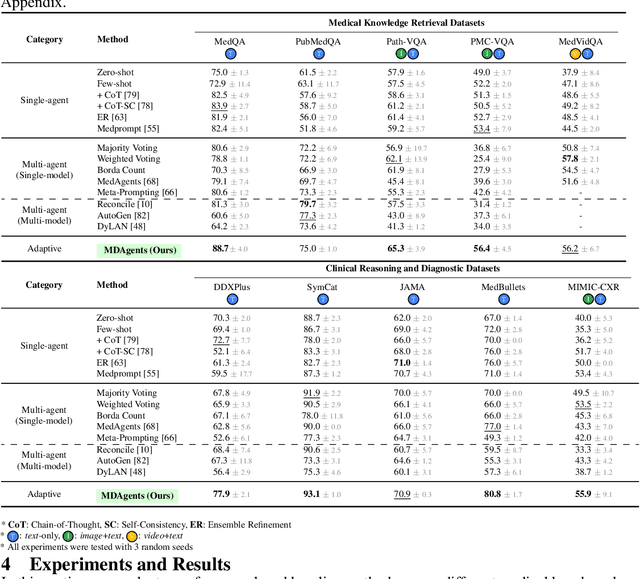
Abstract:Foundation models have become invaluable in advancing the medical field. Despite their promise, the strategic deployment of LLMs for effective utility in complex medical tasks remains an open question. Our novel framework, Medical Decision-making Agents (MDAgents) aims to address this gap by automatically assigning the effective collaboration structure for LLMs. Assigned solo or group collaboration structure is tailored to the complexity of the medical task at hand, emulating real-world medical decision making processes. We evaluate our framework and baseline methods with state-of-the-art LLMs across a suite of challenging medical benchmarks: MedQA, MedMCQA, PubMedQA, DDXPlus, PMC-VQA, Path-VQA, and MedVidQA, achieving the best performance in 5 out of 7 benchmarks that require an understanding of multi-modal medical reasoning. Ablation studies reveal that MDAgents excels in adapting the number of collaborating agents to optimize efficiency and accuracy, showcasing its robustness in diverse scenarios. We also explore the dynamics of group consensus, offering insights into how collaborative agents could behave in complex clinical team dynamics. Our code can be found at https://github.com/mitmedialab/MDAgents.
Recent Advances, Applications, and Open Challenges in Machine Learning for Health: Reflections from Research Roundtables at ML4H 2023 Symposium
Mar 03, 2024Abstract:The third ML4H symposium was held in person on December 10, 2023, in New Orleans, Louisiana, USA. The symposium included research roundtable sessions to foster discussions between participants and senior researchers on timely and relevant topics for the \ac{ML4H} community. Encouraged by the successful virtual roundtables in the previous year, we organized eleven in-person roundtables and four virtual roundtables at ML4H 2022. The organization of the research roundtables at the conference involved 17 Senior Chairs and 19 Junior Chairs across 11 tables. Each roundtable session included invited senior chairs (with substantial experience in the field), junior chairs (responsible for facilitating the discussion), and attendees from diverse backgrounds with interest in the session's topic. Herein we detail the organization process and compile takeaways from these roundtable discussions, including recent advances, applications, and open challenges for each topic. We conclude with a summary and lessons learned across all roundtables. This document serves as a comprehensive review paper, summarizing the recent advancements in machine learning for healthcare as contributed by foremost researchers in the field.
Event-Based Contrastive Learning for Medical Time Series
Dec 16, 2023



Abstract:In clinical practice, one often needs to identify whether a patient is at high risk of adverse outcomes after some key medical event; e.g., the short-term risk of death after an admission for heart failure. This task, however, remains challenging due to the complexity, variability, and heterogeneity of longitudinal medical data, especially for individuals suffering from chronic diseases like heart failure. In this paper, we introduce Event-Based Contrastive Learning (EBCL) - a method for learning embeddings of heterogeneous patient data that preserves temporal information before and after key index events. We demonstrate that EBCL produces models that yield better fine-tuning performance on critical downstream tasks including 30-day readmission, 1-year mortality, and 1-week length of stay relative to other representation learning methods that do not exploit temporal information surrounding key medical events.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge