Rahul Thapa
Stanford Sleep Bench: Evaluating Polysomnography Pre-training Methods for Sleep Foundation Models
Dec 10, 2025Abstract:Polysomnography (PSG), the gold standard test for sleep analysis, generates vast amounts of multimodal clinical data, presenting an opportunity to leverage self-supervised representation learning (SSRL) for pre-training foundation models to enhance sleep analysis. However, progress in sleep foundation models is hindered by two key limitations: (1) the lack of a shared dataset and benchmark with diverse tasks for training and evaluation, and (2) the absence of a systematic evaluation of SSRL approaches across sleep-related tasks. To address these gaps, we introduce Stanford Sleep Bench, a large-scale PSG dataset comprising 17,467 recordings totaling over 163,000 hours from a major sleep clinic, including 13 clinical disease prediction tasks alongside canonical sleep-related tasks such as sleep staging, apnea diagnosis, and age estimation. We systematically evaluate SSRL pre-training methods on Stanford Sleep Bench, assessing downstream performance across four tasks: sleep staging, apnea diagnosis, age estimation, and disease and mortality prediction. Our results show that multiple pretraining methods achieve comparable performance for sleep staging, apnea diagnosis, and age estimation. However, for mortality and disease prediction, contrastive learning significantly outperforms other approaches while also converging faster during pretraining. To facilitate reproducibility and advance sleep research, we will release Stanford Sleep Bench along with pretrained model weights, training pipelines, and evaluation code.
Disentangling Reasoning and Knowledge in Medical Large Language Models
May 16, 2025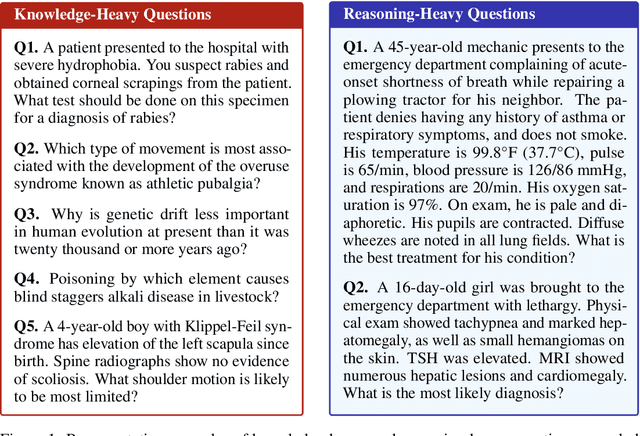
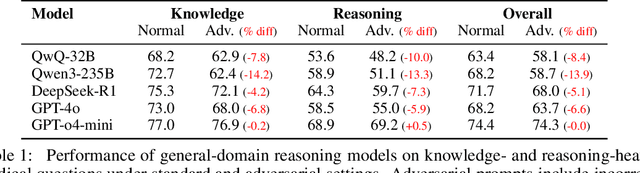
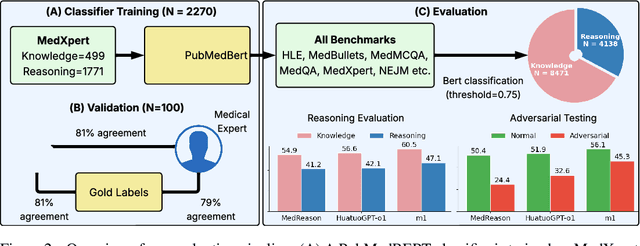
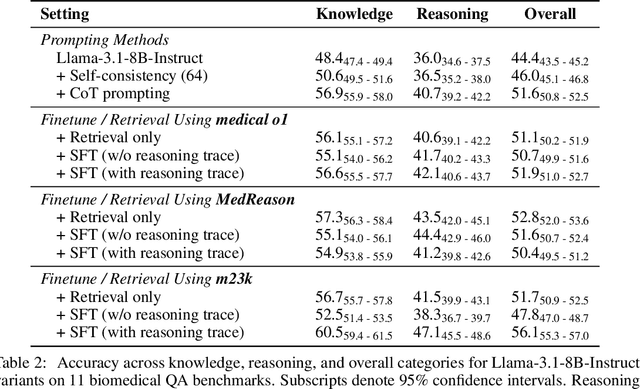
Abstract:Medical reasoning in large language models (LLMs) aims to emulate clinicians' diagnostic thinking, but current benchmarks such as MedQA-USMLE, MedMCQA, and PubMedQA often mix reasoning with factual recall. We address this by separating 11 biomedical QA benchmarks into reasoning- and knowledge-focused subsets using a PubMedBERT classifier that reaches 81 percent accuracy, comparable to human performance. Our analysis shows that only 32.8 percent of questions require complex reasoning. We evaluate biomedical models (HuatuoGPT-o1, MedReason, m1) and general-domain models (DeepSeek-R1, o4-mini, Qwen3), finding consistent gaps between knowledge and reasoning performance. For example, m1 scores 60.5 on knowledge but only 47.1 on reasoning. In adversarial tests where models are misled with incorrect initial reasoning, biomedical models degrade sharply, while larger or RL-trained general models show more robustness. To address this, we train BioMed-R1 using fine-tuning and reinforcement learning on reasoning-heavy examples. It achieves the strongest performance among similarly sized models. Further gains may come from incorporating clinical case reports and training with adversarial and backtracking scenarios.
MedCaseReasoning: Evaluating and learning diagnostic reasoning from clinical case reports
May 16, 2025Abstract:Doctors and patients alike increasingly use Large Language Models (LLMs) to diagnose clinical cases. However, unlike domains such as math or coding, where correctness can be objectively defined by the final answer, medical diagnosis requires both the outcome and the reasoning process to be accurate. Currently, widely used medical benchmarks like MedQA and MMLU assess only accuracy in the final answer, overlooking the quality and faithfulness of the clinical reasoning process. To address this limitation, we introduce MedCaseReasoning, the first open-access dataset for evaluating LLMs on their ability to align with clinician-authored diagnostic reasoning. The dataset includes 14,489 diagnostic question-and-answer cases, each paired with detailed reasoning statements derived from open-access medical case reports. We evaluate state-of-the-art reasoning LLMs on MedCaseReasoning and find significant shortcomings in their diagnoses and reasoning: for instance, the top-performing open-source model, DeepSeek-R1, achieves only 48% 10-shot diagnostic accuracy and mentions only 64% of the clinician reasoning statements (recall). However, we demonstrate that fine-tuning LLMs on the reasoning traces derived from MedCaseReasoning significantly improves diagnostic accuracy and clinical reasoning recall by an average relative gain of 29% and 41%, respectively. The open-source dataset, code, and models are available at https://github.com/kevinwu23/Stanford-MedCaseReasoning.
How Well Can General Vision-Language Models Learn Medicine By Watching Public Educational Videos?
Apr 19, 2025Abstract:Publicly available biomedical videos, such as those on YouTube, serve as valuable educational resources for medical students. Unlike standard machine learning datasets, these videos are designed for human learners, often mixing medical imagery with narration, explanatory diagrams, and contextual framing. In this work, we investigate whether such pedagogically rich, yet non-standardized and heterogeneous videos can effectively teach general-domain vision-language models biomedical knowledge. To this end, we introduce OpenBiomedVi, a biomedical video instruction tuning dataset comprising 1031 hours of video-caption and Q/A pairs, curated through a multi-step human-in-the-loop pipeline. Diverse biomedical video datasets are rare, and OpenBiomedVid fills an important gap by providing instruction-style supervision grounded in real-world educational content. Surprisingly, despite the informal and heterogeneous nature of these videos, the fine-tuned Qwen-2-VL models exhibit substantial performance improvements across most benchmarks. The 2B model achieves gains of 98.7% on video tasks, 71.2% on image tasks, and 0.2% on text tasks. The 7B model shows improvements of 37.09% on video and 11.2% on image tasks, with a slight degradation of 2.7% on text tasks compared to their respective base models. To address the lack of standardized biomedical video evaluation datasets, we also introduce two new expert curated benchmarks, MIMICEchoQA and SurgeryVideoQA. On these benchmarks, the 2B model achieves gains of 99.1% and 98.1%, while the 7B model shows gains of 22.5% and 52.1%, respectively, demonstrating the models' ability to generalize and perform biomedical video understanding on cleaner and more standardized datasets than those seen during training. These results suggest that educational videos created for human learning offer a surprisingly effective training signal for biomedical VLMs.
SMIR: Efficient Synthetic Data Pipeline To Improve Multi-Image Reasoning
Jan 07, 2025Abstract:Vision-Language Models (VLMs) have shown strong performance in understanding single images, aided by numerous high-quality instruction datasets. However, multi-image reasoning tasks are still under-explored in the open-source community due to two main challenges: (1) scaling datasets with multiple correlated images and complex reasoning instructions is resource-intensive and maintaining quality is difficult, and (2) there is a lack of robust evaluation benchmarks for multi-image tasks. To address these issues, we introduce SMIR, an efficient synthetic data-generation pipeline for multi-image reasoning, and a high-quality dataset generated using this pipeline. Our pipeline efficiently extracts highly correlated images using multimodal embeddings, combining visual and descriptive information and leverages open-source LLMs to generate quality instructions. Using this pipeline, we generated 160K synthetic training samples, offering a cost-effective alternative to expensive closed-source solutions. Additionally, we present SMIR-BENCH, a novel multi-image reasoning evaluation benchmark comprising 200 diverse examples across 7 complex multi-image reasoning tasks. SMIR-BENCH is multi-turn and utilizes a VLM judge to evaluate free-form responses, providing a comprehensive assessment of model expressiveness and reasoning capability across modalities. We demonstrate the effectiveness of SMIR dataset by fine-tuning several open-source VLMs and evaluating their performance on SMIR-BENCH. Our results show that models trained on our dataset outperform baseline models in multi-image reasoning tasks up to 8% with a much more scalable data pipeline.
Dragonfly: Multi-Resolution Zoom Supercharges Large Visual-Language Model
Jun 03, 2024



Abstract:Recent advances in large multimodal models (LMMs) suggest that higher image resolution enhances the fine-grained understanding of image details, crucial for tasks such as visual commonsense reasoning and analyzing biomedical images. However, increasing input resolution poses two main challenges: 1) It extends the context length required by the language model, leading to inefficiencies and hitting the model's context limit; 2) It increases the complexity of visual features, necessitating more training data or more complex architecture. We introduce Dragonfly, a new LMM architecture that enhances fine-grained visual understanding and reasoning about image regions to address these challenges. Dragonfly employs two key strategies: multi-resolution visual encoding and zoom-in patch selection. These strategies allow the model to process high-resolution images efficiently while maintaining reasonable context length. Our experiments on eight popular benchmarks demonstrate that Dragonfly achieves competitive or better performance compared to other architectures, highlighting the effectiveness of our design. Additionally, we finetuned Dragonfly on biomedical instructions, achieving state-of-the-art results on multiple biomedical tasks requiring fine-grained visual understanding, including 92.3% accuracy on the Path-VQA dataset (compared to 83.3% for Med-Gemini) and the highest reported results on biomedical image captioning. To support model training, we curated a visual instruction-tuning dataset with 5.5 million image-instruction samples in the general domain and 1.4 million samples in the biomedical domain. We also conducted ablation studies to characterize the impact of various architectural designs and image resolutions, providing insights for future research on visual instruction alignment. The codebase and model are available at https://github.com/togethercomputer/Dragonfly.
SleepFM: Multi-modal Representation Learning for Sleep Across Brain Activity, ECG and Respiratory Signals
May 28, 2024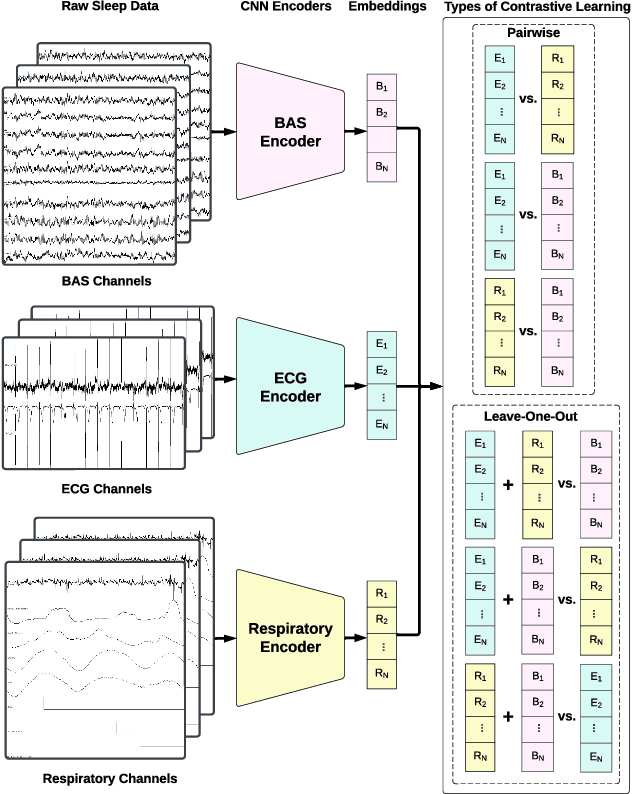
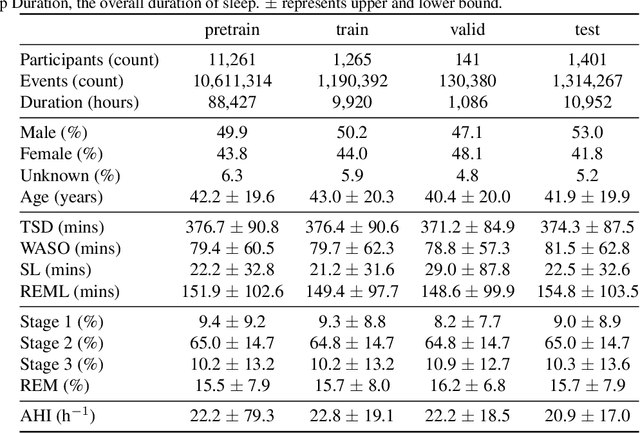
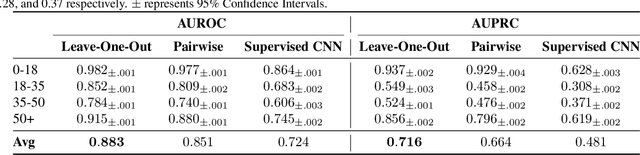
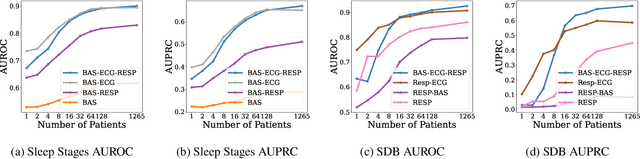
Abstract:Sleep is a complex physiological process evaluated through various modalities recording electrical brain, cardiac, and respiratory activities. We curate a large polysomnography dataset from over 14,000 participants comprising over 100,000 hours of multi-modal sleep recordings. Leveraging this extensive dataset, we developed SleepFM, the first multi-modal foundation model for sleep analysis. We show that a novel leave-one-out approach for contrastive learning significantly improves downstream task performance compared to representations from standard pairwise contrastive learning. A logistic regression model trained on SleepFM's learned embeddings outperforms an end-to-end trained convolutional neural network (CNN) on sleep stage classification (macro AUROC 0.88 vs 0.72 and macro AUPRC 0.72 vs 0.48) and sleep disordered breathing detection (AUROC 0.85 vs 0.69 and AUPRC 0.77 vs 0.61). Notably, the learned embeddings achieve 48% top-1 average accuracy in retrieving the corresponding recording clips of other modalities from 90,000 candidates. This work demonstrates the value of holistic multi-modal sleep modeling to fully capture the richness of sleep recordings. SleepFM is open source and available at https://github.com/rthapa84/sleepfm-codebase.
Standing on FURM ground -- A framework for evaluating Fair, Useful, and Reliable AI Models in healthcare systems
Mar 14, 2024



Abstract:The impact of using artificial intelligence (AI) to guide patient care or operational processes is an interplay of the AI model's output, the decision-making protocol based on that output, and the capacity of the stakeholders involved to take the necessary subsequent action. Estimating the effects of this interplay before deployment, and studying it in real time afterwards, are essential to bridge the chasm between AI model development and achievable benefit. To accomplish this, the Data Science team at Stanford Health Care has developed a Testing and Evaluation (T&E) mechanism to identify fair, useful and reliable AI models (FURM) by conducting an ethical review to identify potential value mismatches, simulations to estimate usefulness, financial projections to assess sustainability, as well as analyses to determine IT feasibility, design a deployment strategy, and recommend a prospective monitoring and evaluation plan. We report on FURM assessments done to evaluate six AI guided solutions for potential adoption, spanning clinical and operational settings, each with the potential to impact from several dozen to tens of thousands of patients each year. We describe the assessment process, summarize the six assessments, and share our framework to enable others to conduct similar assessments. Of the six solutions we assessed, two have moved into a planning and implementation phase. Our novel contributions - usefulness estimates by simulation, financial projections to quantify sustainability, and a process to do ethical assessments - as well as their underlying methods and open source tools, are available for other healthcare systems to conduct actionable evaluations of candidate AI solutions.
OpenMedLM: Prompt engineering can out-perform fine-tuning in medical question-answering with open-source large language models
Feb 29, 2024Abstract:LLMs have become increasingly capable at accomplishing a range of specialized-tasks and can be utilized to expand equitable access to medical knowledge. Most medical LLMs have involved extensive fine-tuning, leveraging specialized medical data and significant, thus costly, amounts of computational power. Many of the top performing LLMs are proprietary and their access is limited to very few research groups. However, open-source (OS) models represent a key area of growth for medical LLMs due to significant improvements in performance and an inherent ability to provide the transparency and compliance required in healthcare. We present OpenMedLM, a prompting platform which delivers state-of-the-art (SOTA) performance for OS LLMs on medical benchmarks. We evaluated a range of OS foundation LLMs (7B-70B) on four medical benchmarks (MedQA, MedMCQA, PubMedQA, MMLU medical-subset). We employed a series of prompting strategies, including zero-shot, few-shot, chain-of-thought (random selection and kNN selection), and ensemble/self-consistency voting. We found that OpenMedLM delivers OS SOTA results on three common medical LLM benchmarks, surpassing the previous best performing OS models that leveraged computationally costly extensive fine-tuning. The model delivers a 72.6% accuracy on the MedQA benchmark, outperforming the previous SOTA by 2.4%, and achieves 81.7% accuracy on the MMLU medical-subset, establishing itself as the first OS LLM to surpass 80% accuracy on this benchmark. Our results highlight medical-specific emergent properties in OS LLMs which have not yet been documented to date elsewhere, and showcase the benefits of further leveraging prompt engineering to improve the performance of accessible LLMs for medical applications.
MedAlign: A Clinician-Generated Dataset for Instruction Following with Electronic Medical Records
Aug 27, 2023
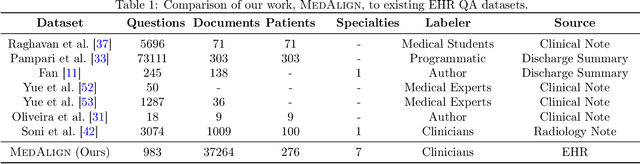
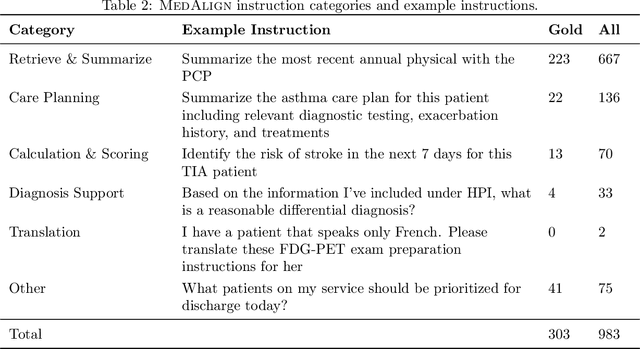

Abstract:The ability of large language models (LLMs) to follow natural language instructions with human-level fluency suggests many opportunities in healthcare to reduce administrative burden and improve quality of care. However, evaluating LLMs on realistic text generation tasks for healthcare remains challenging. Existing question answering datasets for electronic health record (EHR) data fail to capture the complexity of information needs and documentation burdens experienced by clinicians. To address these challenges, we introduce MedAlign, a benchmark dataset of 983 natural language instructions for EHR data. MedAlign is curated by 15 clinicians (7 specialities), includes clinician-written reference responses for 303 instructions, and provides 276 longitudinal EHRs for grounding instruction-response pairs. We used MedAlign to evaluate 6 general domain LLMs, having clinicians rank the accuracy and quality of each LLM response. We found high error rates, ranging from 35% (GPT-4) to 68% (MPT-7B-Instruct), and an 8.3% drop in accuracy moving from 32k to 2k context lengths for GPT-4. Finally, we report correlations between clinician rankings and automated natural language generation metrics as a way to rank LLMs without human review. We make MedAlign available under a research data use agreement to enable LLM evaluations on tasks aligned with clinician needs and preferences.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge