Lanfen Lin
CG-DMER: Hybrid Contrastive-Generative Framework for Disentangled Multimodal ECG Representation Learning
Feb 24, 2026Abstract:Accurate interpretation of electrocardiogram (ECG) signals is crucial for diagnosing cardiovascular diseases. Recent multimodal approaches that integrate ECGs with accompanying clinical reports show strong potential, but they still face two main concerns from a modality perspective: (1) intra-modality: existing models process ECGs in a lead-agnostic manner, overlooking spatial-temporal dependencies across leads, which restricts their effectiveness in modeling fine-grained diagnostic patterns; (2) inter-modality: existing methods directly align ECG signals with clinical reports, introducing modality-specific biases due to the free-text nature of the reports. In light of these two issues, we propose CG-DMER, a contrastive-generative framework for disentangled multimodal ECG representation learning, powered by two key designs: (1) Spatial-temporal masked modeling is designed to better capture fine-grained temporal dynamics and inter-lead spatial dependencies by applying masking across both spatial and temporal dimensions and reconstructing the missing information. (2) A representation disentanglement and alignment strategy is designed to mitigate unnecessary noise and modality-specific biases by introducing modality-specific and modality-shared encoders, ensuring a clearer separation between modality-invariant and modality-specific representations. Experiments on three public datasets demonstrate that CG-DMER achieves state-of-the-art performance across diverse downstream tasks.
MedVAR: Towards Scalable and Efficient Medical Image Generation via Next-scale Autoregressive Prediction
Feb 16, 2026Abstract:Medical image generation is pivotal in applications like data augmentation for low-resource clinical tasks and privacy-preserving data sharing. However, developing a scalable generative backbone for medical imaging requires architectural efficiency, sufficient multi-organ data, and principled evaluation, yet current approaches leave these aspects unresolved. Therefore, we introduce MedVAR, the first autoregressive-based foundation model that adopts the next-scale prediction paradigm to enable fast and scale-up-friendly medical image synthesis. MedVAR generates images in a coarse-to-fine manner and produces structured multi-scale representations suitable for downstream use. To support hierarchical generation, we curate a harmonized dataset of around 440,000 CT and MRI images spanning six anatomical regions. Comprehensive experiments across fidelity, diversity, and scalability show that MedVAR achieves state-of-the-art generative performance and offers a promising architectural direction for future medical generative foundation models.
A Text-Image Fusion Method with Data Augmentation Capabilities for Referring Medical Image Segmentation
Oct 14, 2025



Abstract:Deep learning relies heavily on data augmentation to mitigate limited data, especially in medical imaging. Recent multimodal learning integrates text and images for segmentation, known as referring or text-guided image segmentation. However, common augmentations like rotation and flipping disrupt spatial alignment between image and text, weakening performance. To address this, we propose an early fusion framework that combines text and visual features before augmentation, preserving spatial consistency. We also design a lightweight generator that projects text embeddings into visual space, bridging semantic gaps. Visualization of generated pseudo-images shows accurate region localization. Our method is evaluated on three medical imaging tasks and four segmentation frameworks, achieving state-of-the-art results. Code is publicly available on GitHub: https://github.com/11yxk/MedSeg_EarlyFusion.
EPIC: Efficient Prompt Interaction for Text-Image Classification
Jul 10, 2025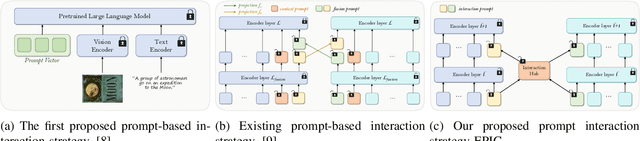
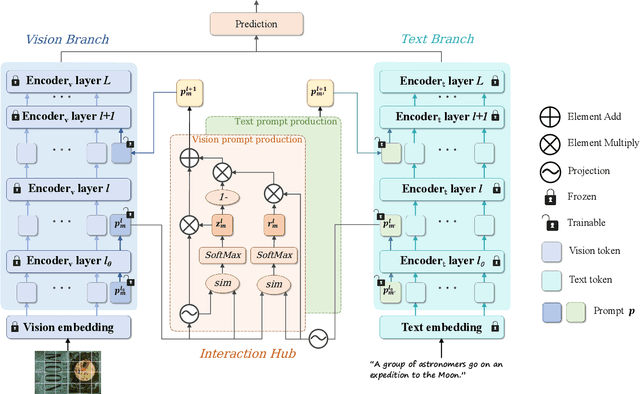
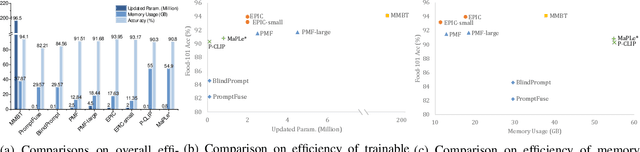
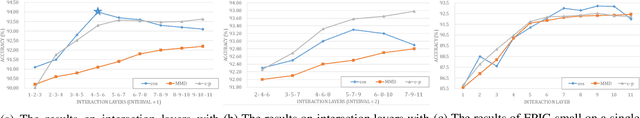
Abstract:In recent years, large-scale pre-trained multimodal models (LMMs) generally emerge to integrate the vision and language modalities, achieving considerable success in multimodal tasks, such as text-image classification. The growing size of LMMs, however, results in a significant computational cost for fine-tuning these models for downstream tasks. Hence, prompt-based interaction strategy is studied to align modalities more efficiently. In this context, we propose a novel efficient prompt-based multimodal interaction strategy, namely Efficient Prompt Interaction for text-image Classification (EPIC). Specifically, we utilize temporal prompts on intermediate layers, and integrate different modalities with similarity-based prompt interaction, to leverage sufficient information exchange between modalities. Utilizing this approach, our method achieves reduced computational resource consumption and fewer trainable parameters (about 1\% of the foundation model) compared to other fine-tuning strategies. Furthermore, it demonstrates superior performance on the UPMC-Food101 and SNLI-VE datasets, while achieving comparable performance on the MM-IMDB dataset.
Enhancing Depression Detection with Chain-of-Thought Prompting: From Emotion to Reasoning Using Large Language Models
Feb 09, 2025Abstract:Depression is one of the leading causes of disability worldwide, posing a severe burden on individuals, healthcare systems, and society at large. Recent advancements in Large Language Models (LLMs) have shown promise in addressing mental health challenges, including the detection of depression through text-based analysis. However, current LLM-based methods often struggle with nuanced symptom identification and lack a transparent, step-by-step reasoning process, making it difficult to accurately classify and explain mental health conditions. To address these challenges, we propose a Chain-of-Thought Prompting approach that enhances both the performance and interpretability of LLM-based depression detection. Our method breaks down the detection process into four stages: (1) sentiment analysis, (2) binary depression classification, (3) identification of underlying causes, and (4) assessment of severity. By guiding the model through these structured reasoning steps, we improve interpretability and reduce the risk of overlooking subtle clinical indicators. We validate our method on the E-DAIC dataset, where we test multiple state-of-the-art large language models. Experimental results indicate that our Chain-of-Thought Prompting technique yields superior performance in both classification accuracy and the granularity of diagnostic insights, compared to baseline approaches.
SemSim: Revisiting Weak-to-Strong Consistency from a Semantic Similarity Perspective for Semi-supervised Medical Image Segmentation
Oct 17, 2024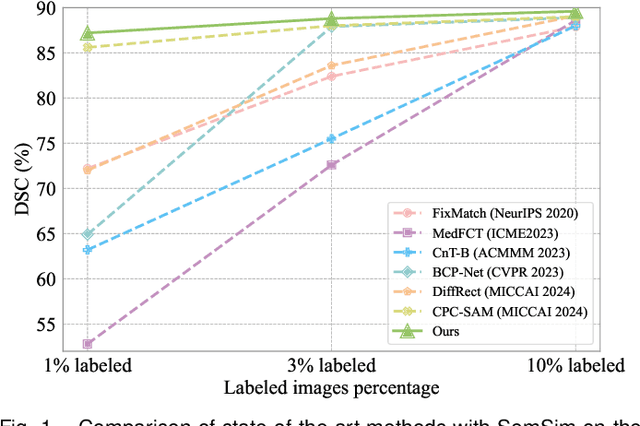
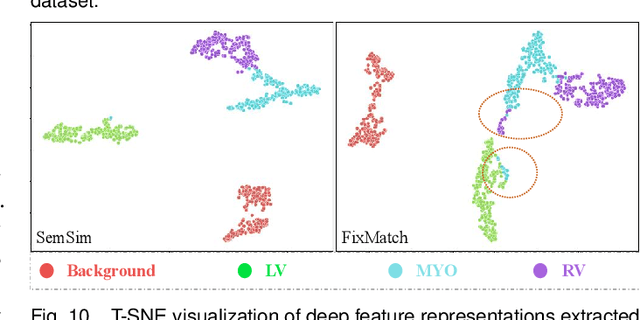
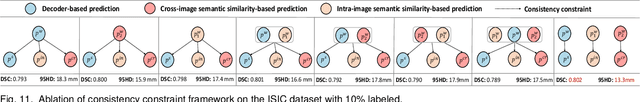
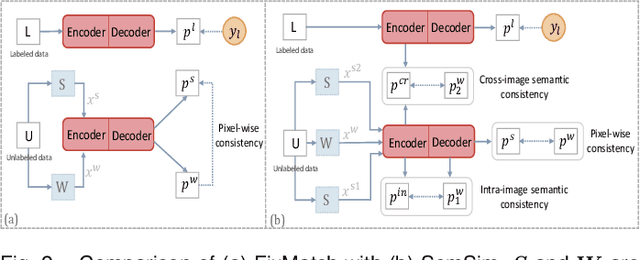
Abstract:Semi-supervised learning (SSL) for medical image segmentation is a challenging yet highly practical task, which reduces reliance on large-scale labeled dataset by leveraging unlabeled samples. Among SSL techniques, the weak-to-strong consistency framework, popularized by FixMatch, has emerged as a state-of-the-art method in classification tasks. Notably, such a simple pipeline has also shown competitive performance in medical image segmentation. However, two key limitations still persist, impeding its efficient adaptation: (1) the neglect of contextual dependencies results in inconsistent predictions for similar semantic features, leading to incomplete object segmentation; (2) the lack of exploitation of semantic similarity between labeled and unlabeled data induces considerable class-distribution discrepancy. To address these limitations, we propose a novel semi-supervised framework based on FixMatch, named SemSim, powered by two appealing designs from semantic similarity perspective: (1) rectifying pixel-wise prediction by reasoning about the intra-image pair-wise affinity map, thus integrating contextual dependencies explicitly into the final prediction; (2) bridging labeled and unlabeled data via a feature querying mechanism for compact class representation learning, which fully considers cross-image anatomical similarities. As the reliable semantic similarity extraction depends on robust features, we further introduce an effective spatial-aware fusion module (SFM) to explore distinctive information from multiple scales. Extensive experiments show that SemSim yields consistent improvements over the state-of-the-art methods across three public segmentation benchmarks.
Deep Self-cleansing for Medical Image Segmentation with Noisy Labels
Sep 08, 2024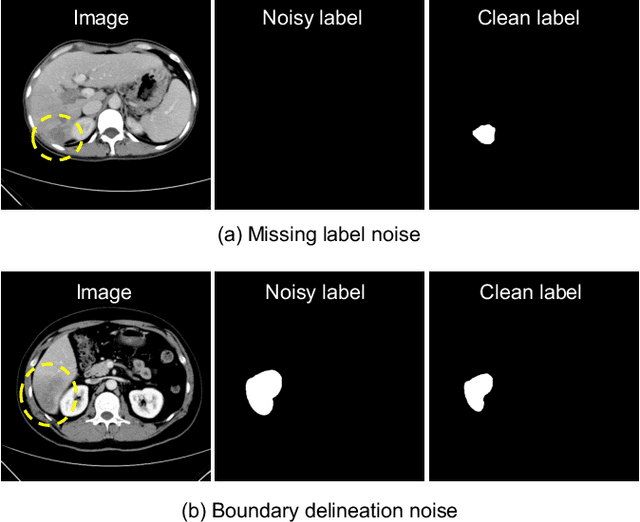
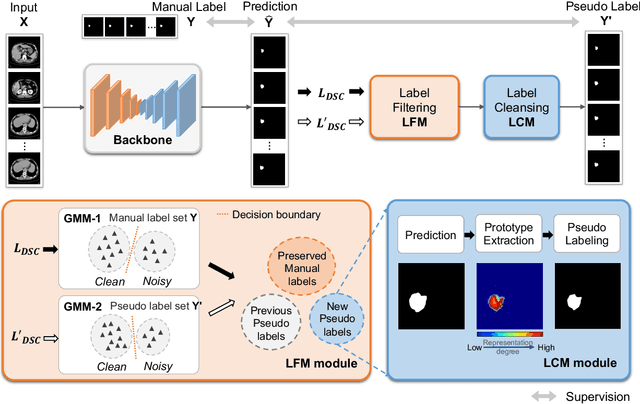
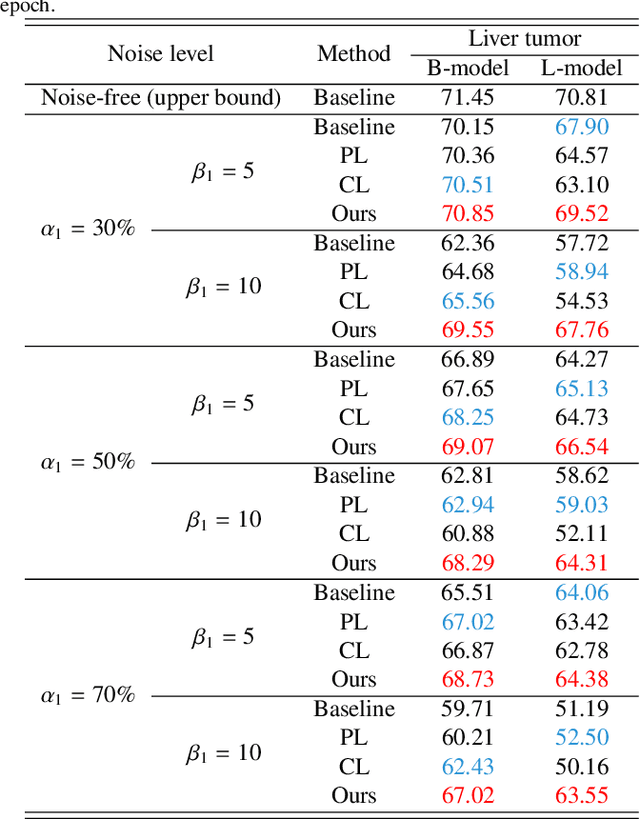
Abstract:Medical image segmentation is crucial in the field of medical imaging, aiding in disease diagnosis and surgical planning. Most established segmentation methods rely on supervised deep learning, in which clean and precise labels are essential for supervision and significantly impact the performance of models. However, manually delineated labels often contain noise, such as missing labels and inaccurate boundary delineation, which can hinder networks from correctly modeling target characteristics. In this paper, we propose a deep self-cleansing segmentation framework that can preserve clean labels while cleansing noisy ones in the training phase. To achieve this, we devise a gaussian mixture model-based label filtering module that distinguishes noisy labels from clean labels. Additionally, we develop a label cleansing module to generate pseudo low-noise labels for identified noisy samples. The preserved clean labels and pseudo-labels are then used jointly to supervise the network. Validated on a clinical liver tumor dataset and a public cardiac diagnosis dataset, our method can effectively suppress the interference from noisy labels and achieve prominent segmentation performance.
LSMS: Language-guided Scale-aware MedSegmentor for Medical Image Referring Segmentation
Sep 02, 2024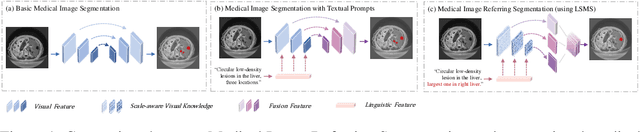
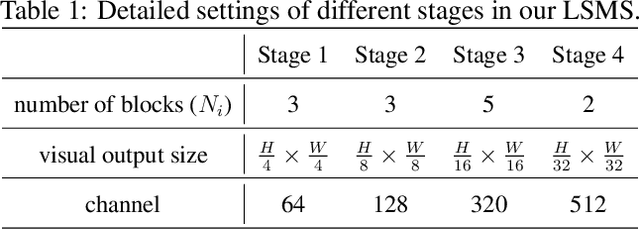
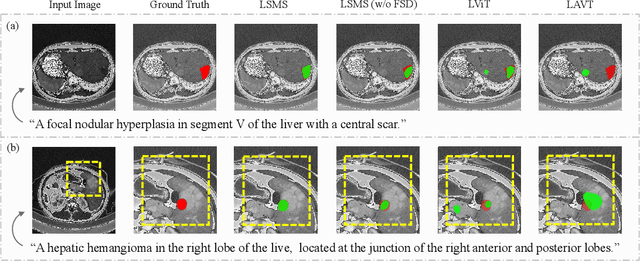
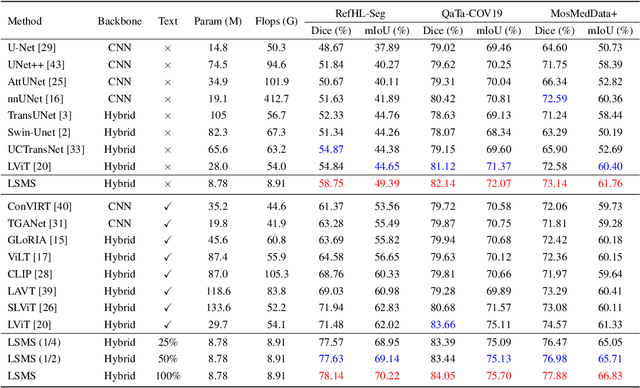
Abstract:Conventional medical image segmentation methods have been found inadequate in facilitating physicians with the identification of specific lesions for diagnosis and treatment. Given the utility of text as an instructional format, we introduce a novel task termed Medical Image Referring Segmentation (MIRS), which requires segmenting specified lesions in images based on the given language expressions. Due to the varying object scales in medical images, MIRS demands robust vision-language modeling and comprehensive multi-scale interaction for precise localization and segmentation under linguistic guidance. However, existing medical image segmentation methods fall short in meeting these demands, resulting in insufficient segmentation accuracy. In response, we propose an approach named Language-guided Scale-aware MedSegmentor (LSMS), incorporating two appealing designs: (1)~a Scale-aware Vision-Language Attention module that leverages diverse convolutional kernels to acquire rich visual knowledge and interact closely with linguistic features, thereby enhancing lesion localization capability; (2)~a Full-Scale Decoder that globally models multi-modal features across various scales, capturing complementary information between scales to accurately outline lesion boundaries. Addressing the lack of suitable datasets for MIRS, we constructed a vision-language medical dataset called Reference Hepatic Lesion Segmentation (RefHL-Seg). This dataset comprises 2,283 abdominal CT slices from 231 cases, with corresponding textual annotations and segmentation masks for various liver lesions in images. We validated the performance of LSMS for MIRS and conventional medical image segmentation tasks across various datasets. Our LSMS consistently outperforms on all datasets with lower computational costs. The code and datasets will be released.
Multitask and Multimodal Neural Tuning for Large Models
Aug 06, 2024



Abstract:In recent years, large-scale multimodal models have demonstrated impressive capabilities across various domains. However, enabling these models to effectively perform multiple multimodal tasks simultaneously remains a significant challenge. To address this, we introduce a novel tuning method called neural tuning, designed to handle diverse multimodal tasks concurrently, including reasoning segmentation, referring segmentation, image captioning, and text-to-image generation. Neural tuning emulates sparse distributed representation in human brain, where only specific subsets of neurons are activated for each task. Additionally, we present a new benchmark, MMUD, where each sample is annotated with multiple task labels. By applying neural tuning to pretrained large models on the MMUD benchmark, we achieve simultaneous task handling in a streamlined and efficient manner. All models, code, and datasets will be publicly available after publication, facilitating further research and development in this field.
HSVLT: Hierarchical Scale-Aware Vision-Language Transformer for Multi-Label Image Classification
Jul 23, 2024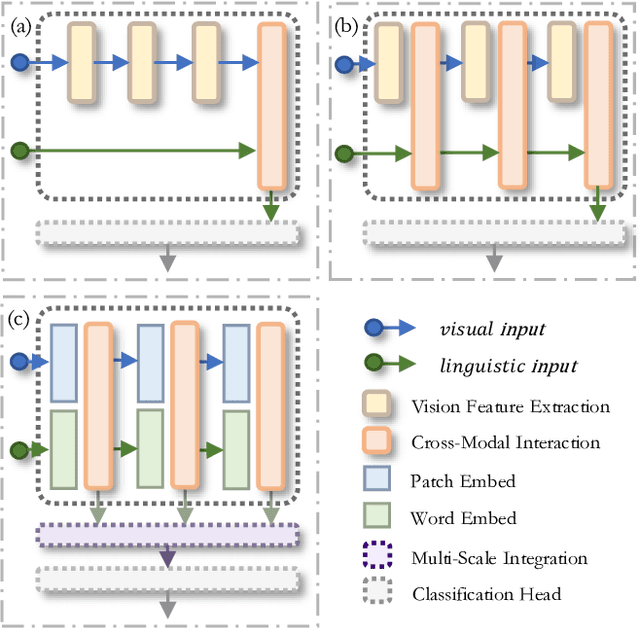
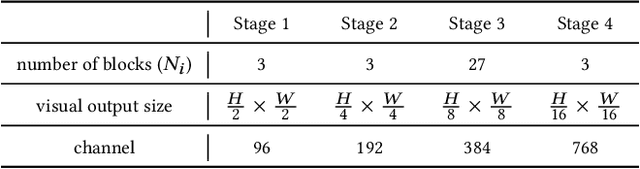
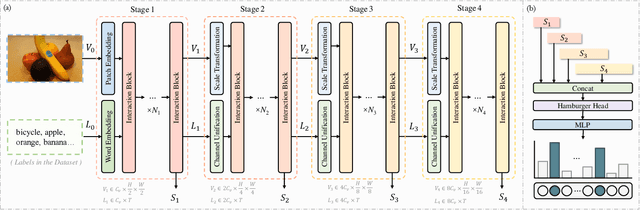
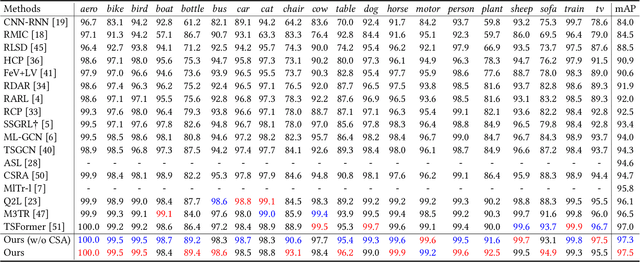
Abstract:The task of multi-label image classification involves recognizing multiple objects within a single image. Considering both valuable semantic information contained in the labels and essential visual features presented in the image, tight visual-linguistic interactions play a vital role in improving classification performance. Moreover, given the potential variance in object size and appearance within a single image, attention to features of different scales can help to discover possible objects in the image. Recently, Transformer-based methods have achieved great success in multi-label image classification by leveraging the advantage of modeling long-range dependencies, but they have several limitations. Firstly, existing methods treat visual feature extraction and cross-modal fusion as separate steps, resulting in insufficient visual-linguistic alignment in the joint semantic space. Additionally, they only extract visual features and perform cross-modal fusion at a single scale, neglecting objects with different characteristics. To address these issues, we propose a Hierarchical Scale-Aware Vision-Language Transformer (HSVLT) with two appealing designs: (1)~A hierarchical multi-scale architecture that involves a Cross-Scale Aggregation module, which leverages joint multi-modal features extracted from multiple scales to recognize objects of varying sizes and appearances in images. (2)~Interactive Visual-Linguistic Attention, a novel attention mechanism module that tightly integrates cross-modal interaction, enabling the joint updating of visual, linguistic and multi-modal features. We have evaluated our method on three benchmark datasets. The experimental results demonstrate that HSVLT surpasses state-of-the-art methods with lower computational cost.
* 10 pages, 6 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge