Shiao Xie
SemSim: Revisiting Weak-to-Strong Consistency from a Semantic Similarity Perspective for Semi-supervised Medical Image Segmentation
Oct 17, 2024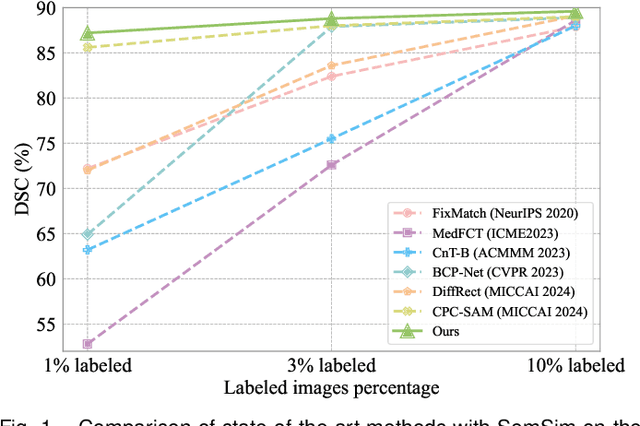
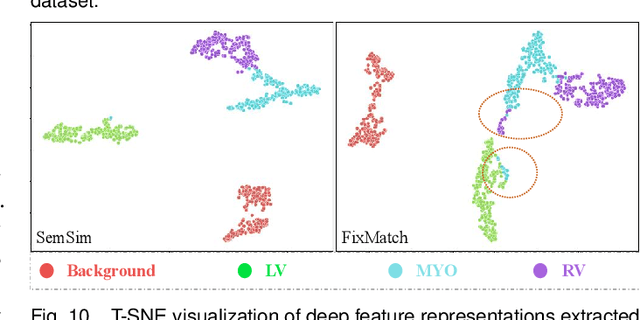
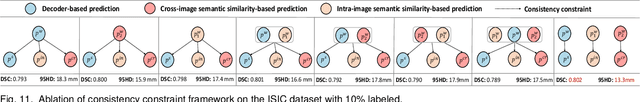
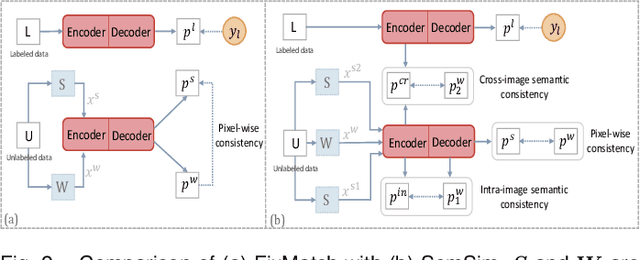
Abstract:Semi-supervised learning (SSL) for medical image segmentation is a challenging yet highly practical task, which reduces reliance on large-scale labeled dataset by leveraging unlabeled samples. Among SSL techniques, the weak-to-strong consistency framework, popularized by FixMatch, has emerged as a state-of-the-art method in classification tasks. Notably, such a simple pipeline has also shown competitive performance in medical image segmentation. However, two key limitations still persist, impeding its efficient adaptation: (1) the neglect of contextual dependencies results in inconsistent predictions for similar semantic features, leading to incomplete object segmentation; (2) the lack of exploitation of semantic similarity between labeled and unlabeled data induces considerable class-distribution discrepancy. To address these limitations, we propose a novel semi-supervised framework based on FixMatch, named SemSim, powered by two appealing designs from semantic similarity perspective: (1) rectifying pixel-wise prediction by reasoning about the intra-image pair-wise affinity map, thus integrating contextual dependencies explicitly into the final prediction; (2) bridging labeled and unlabeled data via a feature querying mechanism for compact class representation learning, which fully considers cross-image anatomical similarities. As the reliable semantic similarity extraction depends on robust features, we further introduce an effective spatial-aware fusion module (SFM) to explore distinctive information from multiple scales. Extensive experiments show that SemSim yields consistent improvements over the state-of-the-art methods across three public segmentation benchmarks.
HSVLT: Hierarchical Scale-Aware Vision-Language Transformer for Multi-Label Image Classification
Jul 23, 2024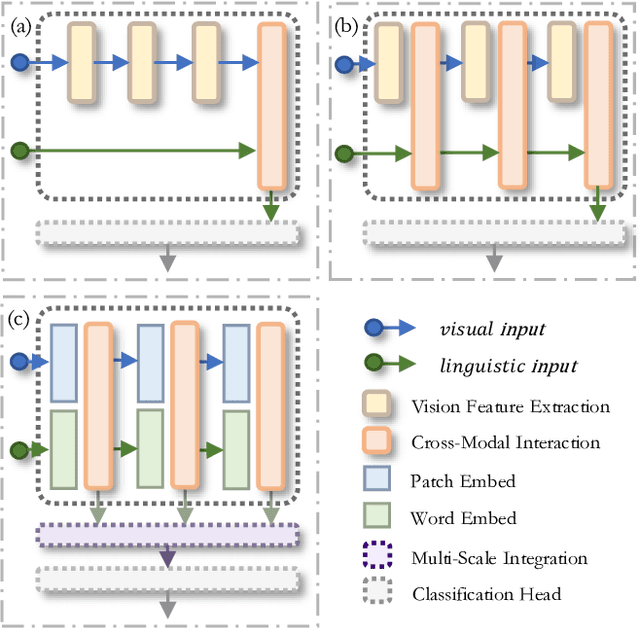
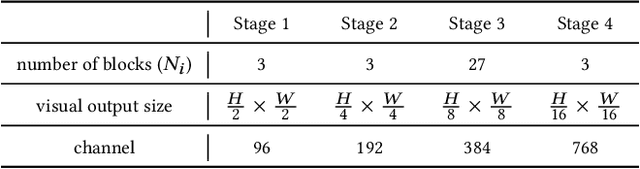
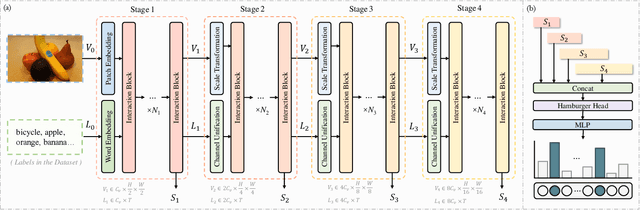
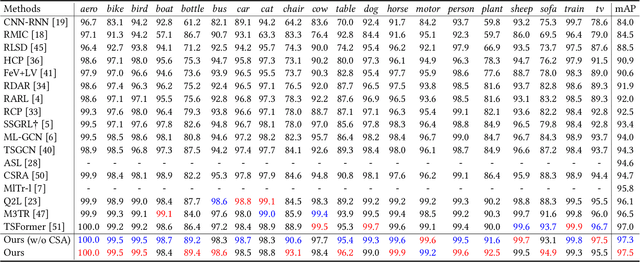
Abstract:The task of multi-label image classification involves recognizing multiple objects within a single image. Considering both valuable semantic information contained in the labels and essential visual features presented in the image, tight visual-linguistic interactions play a vital role in improving classification performance. Moreover, given the potential variance in object size and appearance within a single image, attention to features of different scales can help to discover possible objects in the image. Recently, Transformer-based methods have achieved great success in multi-label image classification by leveraging the advantage of modeling long-range dependencies, but they have several limitations. Firstly, existing methods treat visual feature extraction and cross-modal fusion as separate steps, resulting in insufficient visual-linguistic alignment in the joint semantic space. Additionally, they only extract visual features and perform cross-modal fusion at a single scale, neglecting objects with different characteristics. To address these issues, we propose a Hierarchical Scale-Aware Vision-Language Transformer (HSVLT) with two appealing designs: (1)~A hierarchical multi-scale architecture that involves a Cross-Scale Aggregation module, which leverages joint multi-modal features extracted from multiple scales to recognize objects of varying sizes and appearances in images. (2)~Interactive Visual-Linguistic Attention, a novel attention mechanism module that tightly integrates cross-modal interaction, enabling the joint updating of visual, linguistic and multi-modal features. We have evaluated our method on three benchmark datasets. The experimental results demonstrate that HSVLT surpasses state-of-the-art methods with lower computational cost.
* 10 pages, 6 figures
A Survey on Domain Generalization for Medical Image Analysis
Feb 13, 2024Abstract:Medical Image Analysis (MedIA) has emerged as a crucial tool in computer-aided diagnosis systems, particularly with the advancement of deep learning (DL) in recent years. However, well-trained deep models often experience significant performance degradation when deployed in different medical sites, modalities, and sequences, known as a domain shift issue. In light of this, Domain Generalization (DG) for MedIA aims to address the domain shift challenge by generalizing effectively and performing robustly across unknown data distributions. This paper presents the a comprehensive review of substantial developments in this area. First, we provide a formal definition of domain shift and domain generalization in medical field, and discuss several related settings. Subsequently, we summarize the recent methods from three viewpoints: data manipulation level, feature representation level, and model training level, and present some algorithms in detail for each viewpoints. Furthermore, we introduce the commonly used datasets. Finally, we summarize existing literature and present some potential research topics for the future. For this survey, we also created a GitHub project by collecting the supporting resources, at the link: https://github.com/Ziwei-Niu/DG_for_MedIA
Mixed Transformer U-Net For Medical Image Segmentation
Nov 11, 2021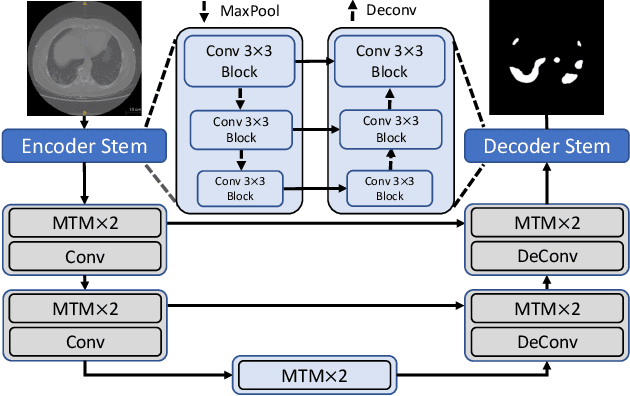
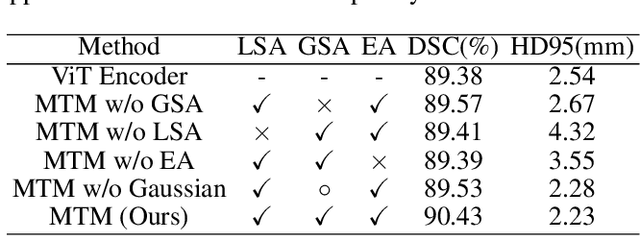
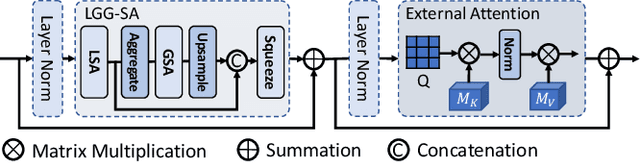
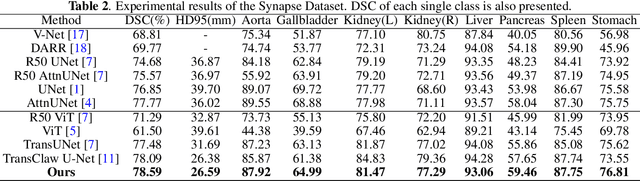
Abstract:Though U-Net has achieved tremendous success in medical image segmentation tasks, it lacks the ability to explicitly model long-range dependencies. Therefore, Vision Transformers have emerged as alternative segmentation structures recently, for their innate ability of capturing long-range correlations through Self-Attention (SA). However, Transformers usually rely on large-scale pre-training and have high computational complexity. Furthermore, SA can only model self-affinities within a single sample, ignoring the potential correlations of the overall dataset. To address these problems, we propose a novel Transformer module named Mixed Transformer Module (MTM) for simultaneous inter- and intra- affinities learning. MTM first calculates self-affinities efficiently through our well-designed Local-Global Gaussian-Weighted Self-Attention (LGG-SA). Then, it mines inter-connections between data samples through External Attention (EA). By using MTM, we construct a U-shaped model named Mixed Transformer U-Net (MT-UNet) for accurate medical image segmentation. We test our method on two different public datasets, and the experimental results show that the proposed method achieves better performance over other state-of-the-art methods. The code is available at: https://github.com/Dootmaan/MT-UNet.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge