Qiuli Wang
From Prompt Optimization to Multi-Dimensional Credibility Evaluation: Enhancing Trustworthiness of Chinese LLM-Generated Liver MRI Reports
Oct 27, 2025Abstract:Large language models (LLMs) have demonstrated promising performance in generating diagnostic conclusions from imaging findings, thereby supporting radiology reporting, trainee education, and quality control. However, systematic guidance on how to optimize prompt design across different clinical contexts remains underexplored. Moreover, a comprehensive and standardized framework for assessing the trustworthiness of LLM-generated radiology reports is yet to be established. This study aims to enhance the trustworthiness of LLM-generated liver MRI reports by introducing a Multi-Dimensional Credibility Assessment (MDCA) framework and providing guidance on institution-specific prompt optimization. The proposed framework is applied to evaluate and compare the performance of several advanced LLMs, including Kimi-K2-Instruct-0905, Qwen3-235B-A22B-Instruct-2507, DeepSeek-V3, and ByteDance-Seed-OSS-36B-Instruct, using the SiliconFlow platform.
Uncertainty-Gated Deformable Network for Breast Tumor Segmentation in MR Images
Sep 19, 2025



Abstract:Accurate segmentation of breast tumors in magnetic resonance images (MRI) is essential for breast cancer diagnosis, yet existing methods face challenges in capturing irregular tumor shapes and effectively integrating local and global features. To address these limitations, we propose an uncertainty-gated deformable network to leverage the complementary information from CNN and Transformers. Specifically, we incorporates deformable feature modeling into both convolution and attention modules, enabling adaptive receptive fields for irregular tumor contours. We also design an Uncertainty-Gated Enhancing Module (U-GEM) to selectively exchange complementary features between CNN and Transformer based on pixel-wise uncertainty, enhancing both local and global representations. Additionally, a Boundary-sensitive Deep Supervision Loss is introduced to further improve tumor boundary delineation. Comprehensive experiments on two clinical breast MRI datasets demonstrate that our method achieves superior segmentation performance compared with state-of-the-art methods, highlighting its clinical potential for accurate breast tumor delineation.
Deep Self-cleansing for Medical Image Segmentation with Noisy Labels
Sep 08, 2024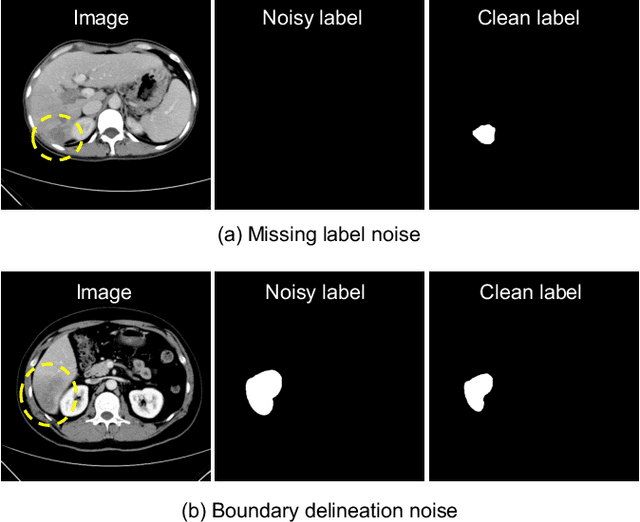
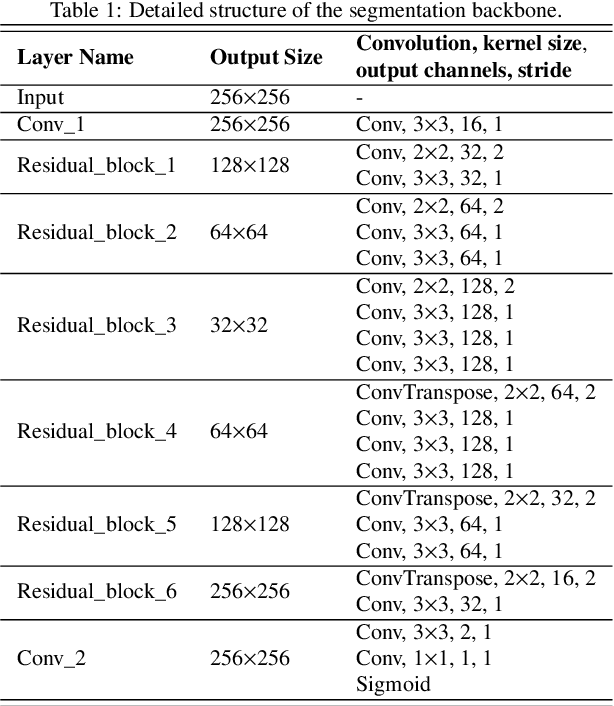
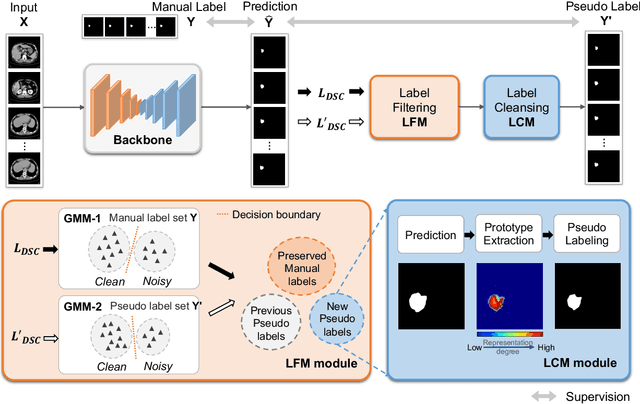
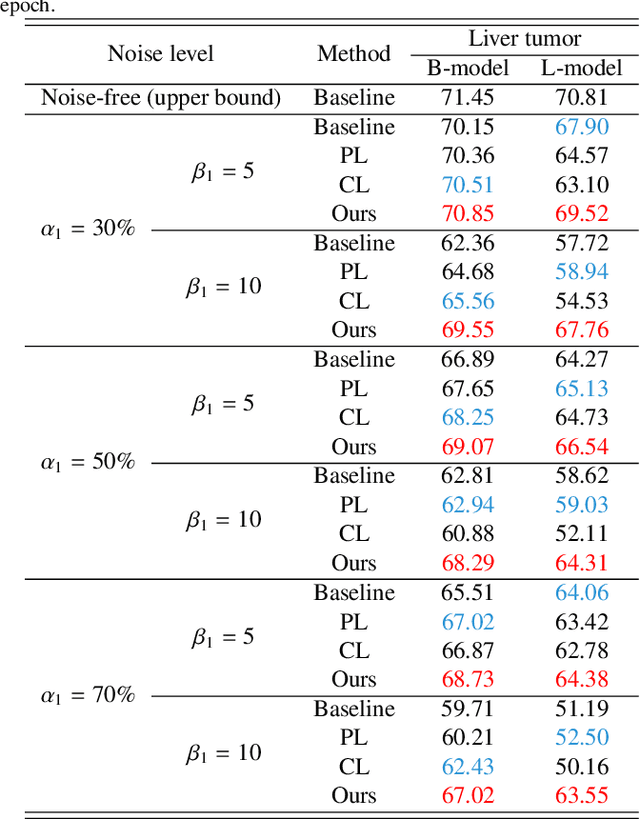
Abstract:Medical image segmentation is crucial in the field of medical imaging, aiding in disease diagnosis and surgical planning. Most established segmentation methods rely on supervised deep learning, in which clean and precise labels are essential for supervision and significantly impact the performance of models. However, manually delineated labels often contain noise, such as missing labels and inaccurate boundary delineation, which can hinder networks from correctly modeling target characteristics. In this paper, we propose a deep self-cleansing segmentation framework that can preserve clean labels while cleansing noisy ones in the training phase. To achieve this, we devise a gaussian mixture model-based label filtering module that distinguishes noisy labels from clean labels. Additionally, we develop a label cleansing module to generate pseudo low-noise labels for identified noisy samples. The preserved clean labels and pseudo-labels are then used jointly to supervise the network. Validated on a clinical liver tumor dataset and a public cardiac diagnosis dataset, our method can effectively suppress the interference from noisy labels and achieve prominent segmentation performance.
Histology Virtual Staining with Mask-Guided Adversarial Transfer Learning for Tertiary Lymphoid Structure Detection
Aug 26, 2024Abstract:Histological Tertiary Lymphoid Structures (TLSs) are increasingly recognized for their correlation with the efficacy of immunotherapy in various solid tumors. Traditionally, the identification and characterization of TLSs rely on immunohistochemistry (IHC) staining techniques, utilizing markers such as CD20 for B cells. Despite the specificity of IHC, Hematoxylin-Eosin (H&E) staining offers a more accessible and cost-effective choice. Capitalizing on the prevalence of H&E staining slides, we introduce a novel Mask-Guided Adversarial Transfer Learning method designed for virtual pathological staining. This method adeptly captures the nuanced color variations across diverse tissue types under various staining conditions, such as nucleus, red blood cells, positive reaction regions, without explicit label information, and adeptly synthesizes realistic IHC-like virtual staining patches, even replicating the positive reaction. Further, we propose the Virtual IHC Pathology Analysis Network (VIPA-Net), an integrated framework encompassing a Mask-Guided Transfer Module and an H&E-Based Virtual Staining TLS Detection Module. VIPA-Net synergistically harnesses both H\&E staining slides and the synthesized virtual IHC patches to enhance the detection of TLSs within H&E Whole Slide Images (WSIs). We evaluate the network with a comprehensive dataset comprising 1019 annotated slides from The Cancer Genome Atlas (TCGA). Experimental results compellingly illustrate that the VIPA-Net substantially elevates TLS detection accuracy, effectively circumventing the need for actual CD20 staining across the public dataset.
Unified Multi-Modal Image Synthesis for Missing Modality Imputation
Apr 11, 2023



Abstract:Multi-modal medical images provide complementary soft-tissue characteristics that aid in the screening and diagnosis of diseases. However, limited scanning time, image corruption and various imaging protocols often result in incomplete multi-modal images, thus limiting the usage of multi-modal data for clinical purposes. To address this issue, in this paper, we propose a novel unified multi-modal image synthesis method for missing modality imputation. Our method overall takes a generative adversarial architecture, which aims to synthesize missing modalities from any combination of available ones with a single model. To this end, we specifically design a Commonality- and Discrepancy-Sensitive Encoder for the generator to exploit both modality-invariant and specific information contained in input modalities. The incorporation of both types of information facilitates the generation of images with consistent anatomy and realistic details of the desired distribution. Besides, we propose a Dynamic Feature Unification Module to integrate information from a varying number of available modalities, which enables the network to be robust to random missing modalities. The module performs both hard integration and soft integration, ensuring the effectiveness of feature combination while avoiding information loss. Verified on two public multi-modal magnetic resonance datasets, the proposed method is effective in handling various synthesis tasks and shows superior performance compared to previous methods.
Lung Nodule Segmentation and Low-Confidence Region Prediction with Uncertainty-Aware Attention Mechanism
Mar 19, 2023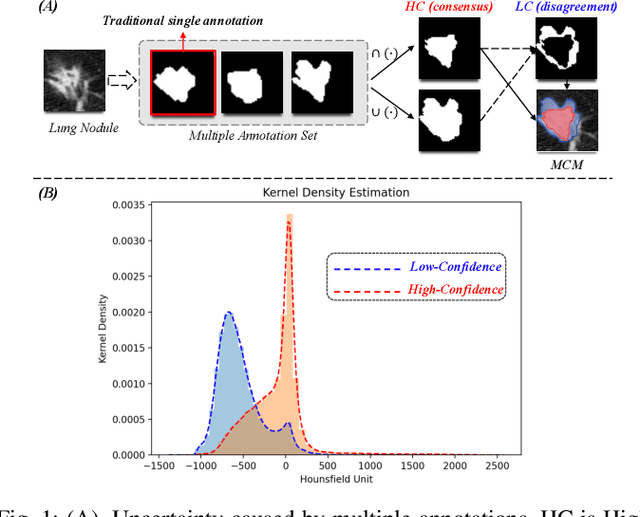
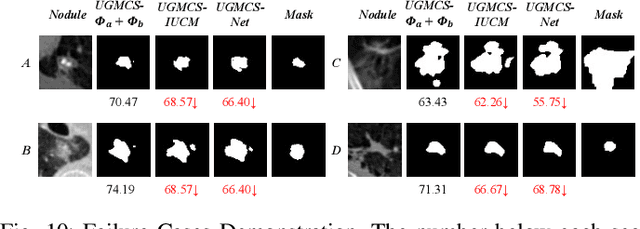
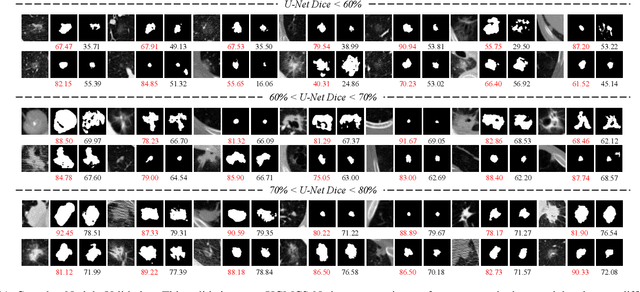
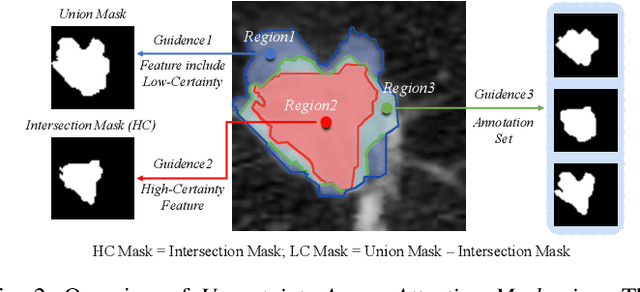
Abstract:Radiologists have different training and clinical experiences, so they may provide various segmentation annotations for a lung nodule, which causes segmentation uncertainty among multiple annotations. Conventional methods usually chose a single annotation as the learning target or tried to learn a latent space of various annotations. Still, they wasted the valuable information of consensus or disagreements ingrained in the multiple annotations. This paper proposes an Uncertainty-Aware Attention Mechanism (UAAM), which utilizes consensus or disagreements among annotations to produce a better segmentation. In UAAM, we propose a Multi-Confidence Mask (MCM), which is a combination of a Low-Confidence (LC) Mask and a High-Confidence (HC) Mask. LC mask indicates regions with low segmentation confidence, which may cause different segmentation options among radiologists. Following UAAM, we further design an Uncertainty-Guide Segmentation Network (UGS-Net), which contains three modules:Feature Extracting Module captures a general feature of a lung nodule. Uncertainty-Aware Module produce three features for the annotations' union, intersection, and annotation set. Finally, Intersection-Union Constraining Module use distances between three features to balance the predictions of final segmentation, LC mask, and HC mask. To fully demonstrate the performance of our method, we propose a Complex Nodule Challenge on LIDC-IDRI, which tests UGS-Net's segmentation performance on the lung nodules that are difficult to segment by U-Net. Experimental results demonstrate that our method can significantly improve the segmentation performance on nodules with poor segmentation by U-Net.
Dual Windows Are Significant: Learning from Mediastinal Window and Focusing on Lung Window
Jun 08, 2022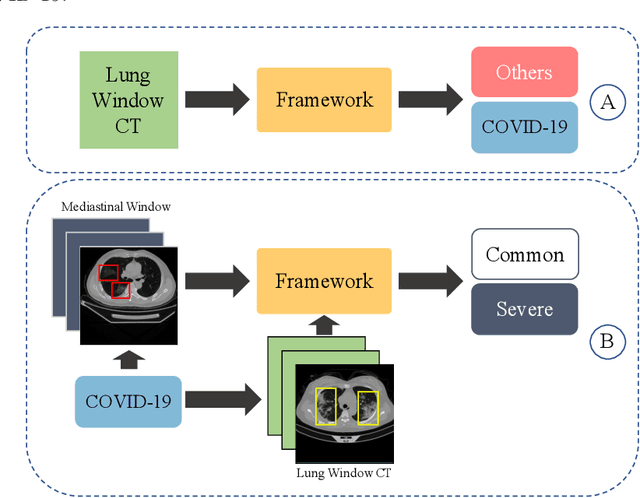

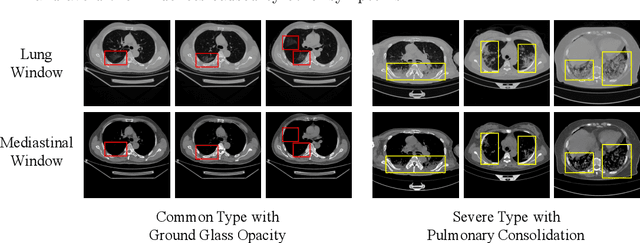
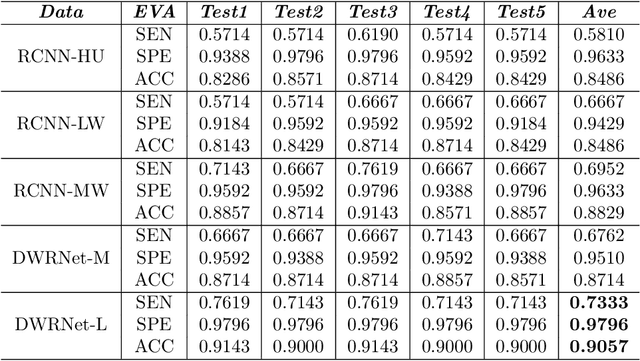
Abstract:Since the pandemic of COVID-19, several deep learning methods were proposed to analyze the chest Computed Tomography (CT) for diagnosis. In the current situation, the disease course classification is significant for medical personnel to decide the treatment. Most previous deep-learning-based methods extract features observed from the lung window. However, it has been proved that some appearances related to diagnosis can be observed better from the mediastinal window rather than the lung window, e.g., the pulmonary consolidation happens more in severe symptoms. In this paper, we propose a novel Dual Window RCNN Network (DWRNet), which mainly learns the distinctive features from the successive mediastinal window. Regarding the features extracted from the lung window, we introduce the Lung Window Attention Block (LWA Block) to pay additional attention to them for enhancing the mediastinal-window features. Moreover, instead of picking up specific slices from the whole CT slices, we use a Recurrent CNN and analyze successive slices as videos. Experimental results show that the fused and representative features improve the predictions of disease course by reaching the accuracy of 90.57%, against the baseline with an accuracy of 84.86%. Ablation studies demonstrate that combined dual window features are more efficient than lung-window features alone, while paying attention to lung-window features can improve the model's stability.
Uncertainty-Aware Lung Nodule Segmentation with Multiple Annotations
Oct 24, 2021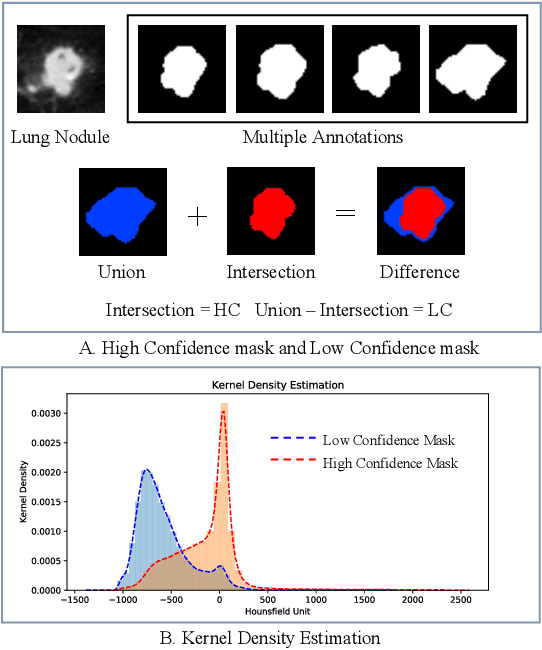
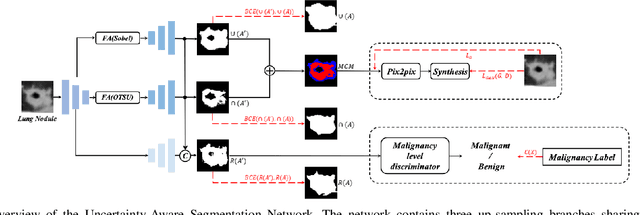
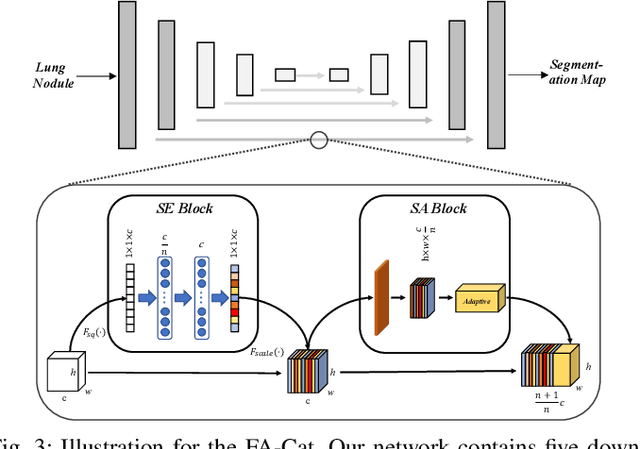
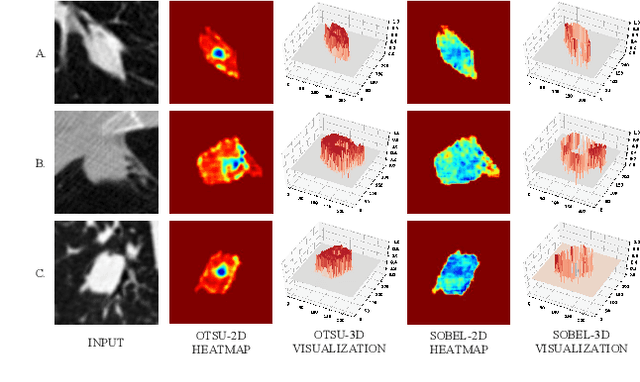
Abstract:Since radiologists have different training and clinical experience, they may provide various segmentation maps for a lung nodule. As a result, for a specific lung nodule, some regions have a higher chance of causing segmentation uncertainty, which brings difficulty for lung nodule segmentation with multiple annotations. To address this problem, this paper proposes an Uncertainty-Aware Segmentation Network (UAS-Net) based on multi-branch U-Net, which can learn the valuable visual features from the regions that may cause segmentation uncertainty and contribute to a better segmentation result. Meanwhile, this network can provide a Multi-Confidence Mask (MCM) simultaneously, pointing out regions with different segmentation uncertainty levels. We introduce a Feature-Aware Concatenation structure for different learning targets and let each branch have a specific learning preference. Moreover, a joint adversarial learning process is also adopted to help learn discriminative features of complex structures. Experimental results show that our method can predict the reasonable regions with higher uncertainty and improve lung nodule segmentation performance in LIDC-IDRI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge