Imon Banerjee
Feature Quality and Adaptability of Medical Foundation Models: A Comparative Evaluation for Radiographic Classification and Segmentation
Nov 12, 2025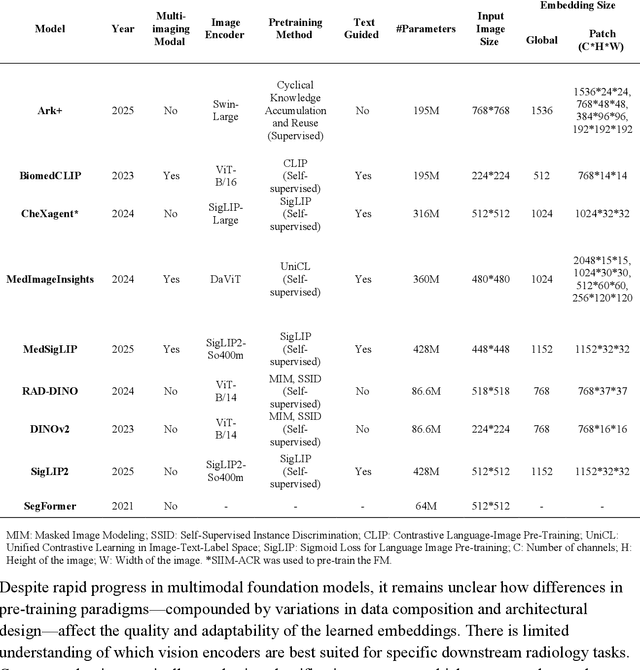
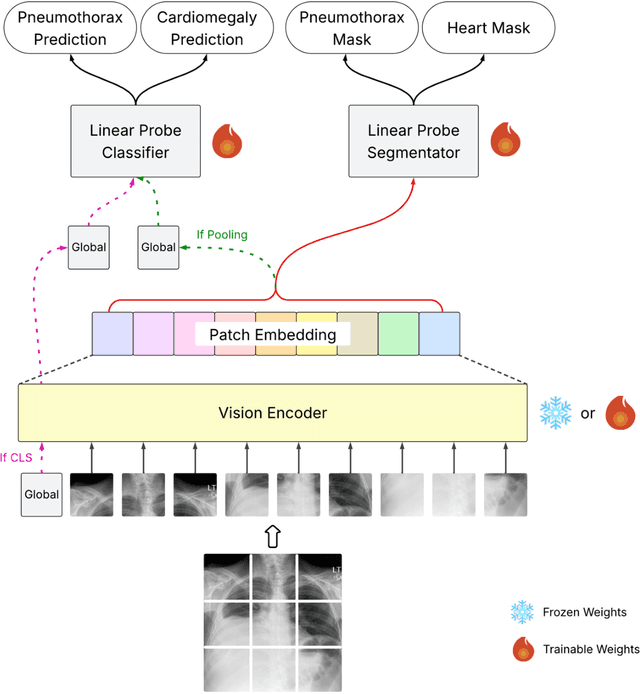
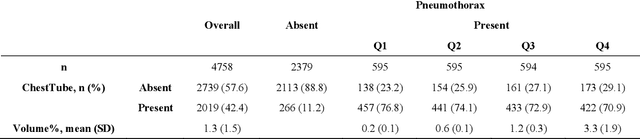
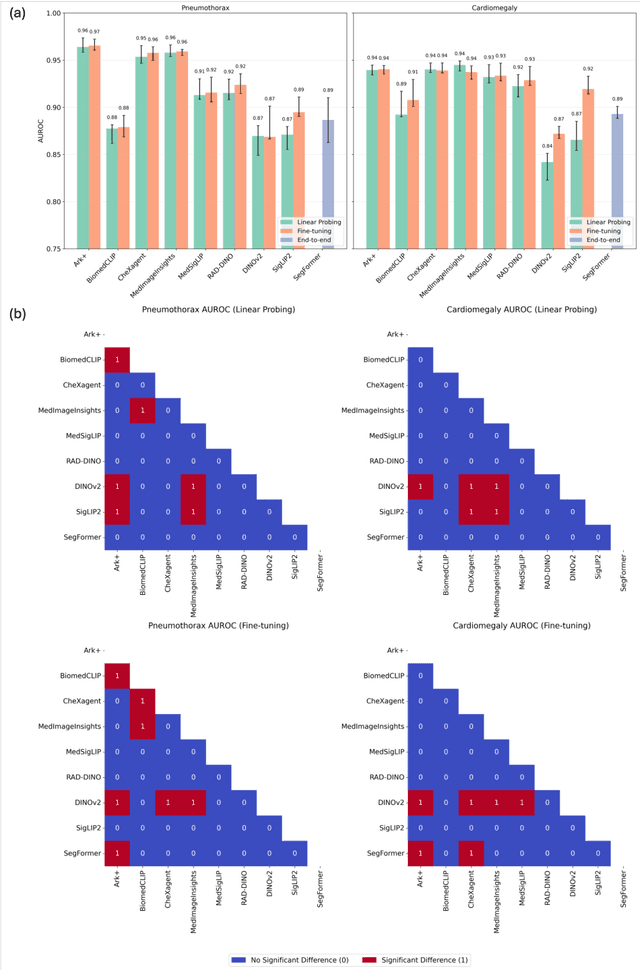
Abstract:Foundation models (FMs) promise to generalize medical imaging, but their effectiveness varies. It remains unclear how pre-training domain (medical vs. general), paradigm (e.g., text-guided), and architecture influence embedding quality, hindering the selection of optimal encoders for specific radiology tasks. To address this, we evaluate vision encoders from eight medical and general-domain FMs for chest X-ray analysis. We benchmark classification (pneumothorax, cardiomegaly) and segmentation (pneumothorax, cardiac boundary) using linear probing and fine-tuning. Our results show that domain-specific pre-training provides a significant advantage; medical FMs consistently outperformed general-domain models in linear probing, establishing superior initial feature quality. However, feature utility is highly task-dependent. Pre-trained embeddings were strong for global classification and segmenting salient anatomy (e.g., heart). In contrast, for segmenting complex, subtle pathologies (e.g., pneumothorax), all FMs performed poorly without significant fine-tuning, revealing a critical gap in localizing subtle disease. Subgroup analysis showed FMs use confounding shortcuts (e.g., chest tubes for pneumothorax) for classification, a strategy that fails for precise segmentation. We also found that expensive text-image alignment is not a prerequisite; image-only (RAD-DINO) and label-supervised (Ark+) FMs were among top performers. Notably, a supervised, end-to-end baseline remained highly competitive, matching or exceeding the best FMs on segmentation tasks. These findings show that while medical pre-training is beneficial, architectural choices (e.g., multi-scale) are critical, and pre-trained features are not universally effective, especially for complex localization tasks where supervised models remain a strong alternative.
MOSCARD -- Causal Reasoning and De-confounding for Multimodal Opportunistic Screening of Cardiovascular Adverse Events
Jun 23, 2025
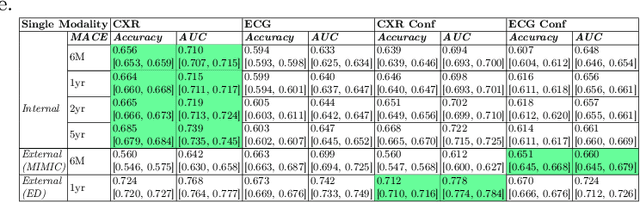


Abstract:Major Adverse Cardiovascular Events (MACE) remain the leading cause of mortality globally, as reported in the Global Disease Burden Study 2021. Opportunistic screening leverages data collected from routine health check-ups and multimodal data can play a key role to identify at-risk individuals. Chest X-rays (CXR) provide insights into chronic conditions contributing to major adverse cardiovascular events (MACE), while 12-lead electrocardiogram (ECG) directly assesses cardiac electrical activity and structural abnormalities. Integrating CXR and ECG could offer a more comprehensive risk assessment than conventional models, which rely on clinical scores, computed tomography (CT) measurements, or biomarkers, which may be limited by sampling bias and single modality constraints. We propose a novel predictive modeling framework - MOSCARD, multimodal causal reasoning with co-attention to align two distinct modalities and simultaneously mitigate bias and confounders in opportunistic risk estimation. Primary technical contributions are - (i) multimodal alignment of CXR with ECG guidance; (ii) integration of causal reasoning; (iii) dual back-propagation graph for de-confounding. Evaluated on internal, shift data from emergency department (ED) and external MIMIC datasets, our model outperformed single modality and state-of-the-art foundational models - AUC: 0.75, 0.83, 0.71 respectively. Proposed cost-effective opportunistic screening enables early intervention, improving patient outcomes and reducing disparities.
CXR-LT 2024: A MICCAI challenge on long-tailed, multi-label, and zero-shot disease classification from chest X-ray
Jun 09, 2025Abstract:The CXR-LT series is a community-driven initiative designed to enhance lung disease classification using chest X-rays (CXR). It tackles challenges in open long-tailed lung disease classification and enhances the measurability of state-of-the-art techniques. The first event, CXR-LT 2023, aimed to achieve these goals by providing high-quality benchmark CXR data for model development and conducting comprehensive evaluations to identify ongoing issues impacting lung disease classification performance. Building on the success of CXR-LT 2023, the CXR-LT 2024 expands the dataset to 377,110 chest X-rays (CXRs) and 45 disease labels, including 19 new rare disease findings. It also introduces a new focus on zero-shot learning to address limitations identified in the previous event. Specifically, CXR-LT 2024 features three tasks: (i) long-tailed classification on a large, noisy test set, (ii) long-tailed classification on a manually annotated "gold standard" subset, and (iii) zero-shot generalization to five previously unseen disease findings. This paper provides an overview of CXR-LT 2024, detailing the data curation process and consolidating state-of-the-art solutions, including the use of multimodal models for rare disease detection, advanced generative approaches to handle noisy labels, and zero-shot learning strategies for unseen diseases. Additionally, the expanded dataset enhances disease coverage to better represent real-world clinical settings, offering a valuable resource for future research. By synthesizing the insights and innovations of participating teams, we aim to advance the development of clinically realistic and generalizable diagnostic models for chest radiography.
CLT and Edgeworth Expansion for m-out-of-n Bootstrap Estimators of The Studentized Median
May 16, 2025Abstract:The m-out-of-n bootstrap, originally proposed by Bickel, Gotze, and Zwet (1992), approximates the distribution of a statistic by repeatedly drawing m subsamples (with m much smaller than n) without replacement from an original sample of size n. It is now routinely used for robust inference with heavy-tailed data, bandwidth selection, and other large-sample applications. Despite its broad applicability across econometrics, biostatistics, and machine learning, rigorous parameter-free guarantees for the soundness of the m-out-of-n bootstrap when estimating sample quantiles have remained elusive. This paper establishes such guarantees by analyzing the estimator of sample quantiles obtained from m-out-of-n resampling of a dataset of size n. We first prove a central limit theorem for a fully data-driven version of the estimator that holds under a mild moment condition and involves no unknown nuisance parameters. We then show that the moment assumption is essentially tight by constructing a counter-example in which the CLT fails. Strengthening the assumptions slightly, we derive an Edgeworth expansion that provides exact convergence rates and, as a corollary, a Berry Esseen bound on the bootstrap approximation error. Finally, we illustrate the scope of our results by deriving parameter-free asymptotic distributions for practical statistics, including the quantiles for random walk Metropolis-Hastings and the rewards of ergodic Markov decision processes, thereby demonstrating the usefulness of our theory in modern estimation and learning tasks.
Position: Restructuring of Categories and Implementation of Guidelines Essential for VLM Adoption in Healthcare
May 12, 2025Abstract:The intricate and multifaceted nature of vision language model (VLM) development, adaptation, and application necessitates the establishment of clear and standardized reporting protocols, particularly within the high-stakes context of healthcare. Defining these reporting standards is inherently challenging due to the diverse nature of studies involving VLMs, which vary significantly from the development of all new VLMs or finetuning for domain alignment to off-the-shelf use of VLM for targeted diagnosis and prediction tasks. In this position paper, we argue that traditional machine learning reporting standards and evaluation guidelines must be restructured to accommodate multiphase VLM studies; it also has to be organized for intuitive understanding of developers while maintaining rigorous standards for reproducibility. To facilitate community adoption, we propose a categorization framework for VLM studies and outline corresponding reporting standards that comprehensively address performance evaluation, data reporting protocols, and recommendations for manuscript composition. These guidelines are organized according to the proposed categorization scheme. Lastly, we present a checklist that consolidates reporting standards, offering a standardized tool to ensure consistency and quality in the publication of VLM-related research.
Opportunistic Screening for Pancreatic Cancer using Computed Tomography Imaging and Radiology Reports
Mar 31, 2025Abstract:Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer, with most cases diagnosed at stage IV and a five-year overall survival rate below 5%. Early detection and prognosis modeling are crucial for improving patient outcomes and guiding early intervention strategies. In this study, we developed and evaluated a deep learning fusion model that integrates radiology reports and CT imaging to predict PDAC risk. The model achieved a concordance index (C-index) of 0.6750 (95% CI: 0.6429, 0.7121) and 0.6435 (95% CI: 0.6055, 0.6789) on the internal and external dataset, respectively, for 5-year survival risk estimation. Kaplan-Meier analysis demonstrated significant separation (p<0.0001) between the low and high risk groups predicted by the fusion model. These findings highlight the potential of deep learning-based survival models in leveraging clinical and imaging data for pancreatic cancer.
Novel AI-Based Quantification of Breast Arterial Calcification to Predict Cardiovascular Risk
Mar 17, 2025Abstract:Women are underdiagnosed and undertreated for cardiovascular disease. Automatic quantification of breast arterial calcification on screening mammography can identify women at risk for cardiovascular disease and enable earlier treatment and management of disease. In this retrospective study of 116,135 women from two healthcare systems, a transformer-based neural network quantified BAC severity (no BAC, mild, moderate, and severe) on screening mammograms. Outcomes included major adverse cardiovascular events (MACE) and all-cause mortality. BAC severity was independently associated with MACE after adjusting for cardiovascular risk factors, with increasing hazard ratios from mild (HR 1.18-1.22), moderate (HR 1.38-1.47), to severe BAC (HR 2.03-2.22) across datasets (all p<0.001). This association remained significant across all age groups, with even mild BAC indicating increased risk in women under 50. BAC remained an independent predictor when analyzed alongside ASCVD risk scores, showing significant associations with myocardial infarction, stroke, heart failure, and mortality (all p<0.005). Automated BAC quantification enables opportunistic cardiovascular risk assessment during routine mammography without additional radiation or cost. This approach provides value beyond traditional risk factors, particularly in younger women, offering potential for early CVD risk stratification in the millions of women undergoing annual mammography.
Unsupervised Hybrid framework for ANomaly Detection (HAND) -- applied to Screening Mammogram
Sep 17, 2024
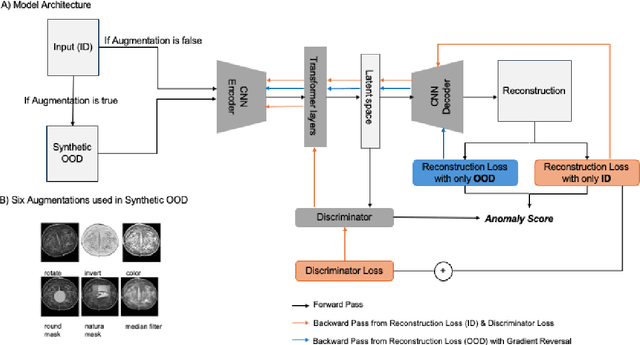
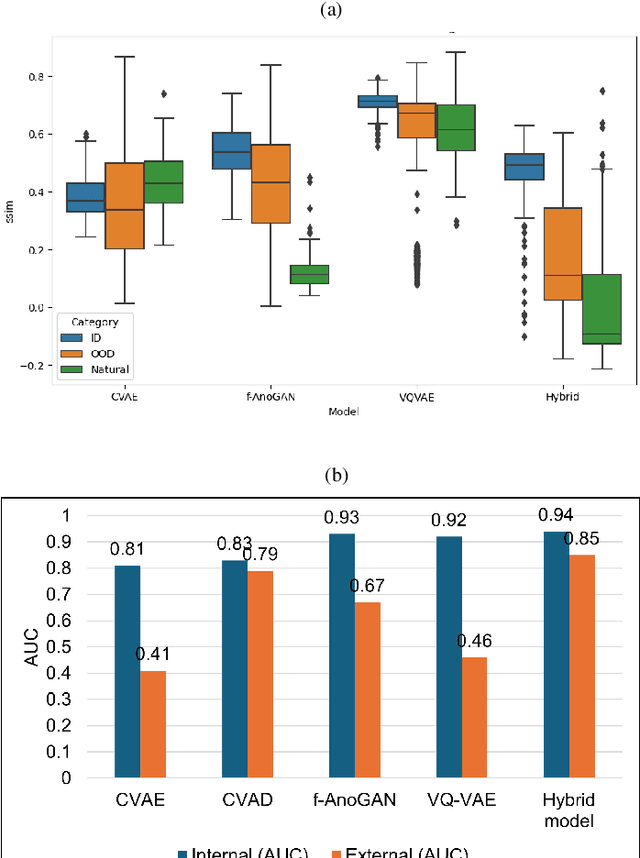
Abstract:Out-of-distribution (OOD) detection is crucial for enhancing the generalization of AI models used in mammogram screening. Given the challenge of limited prior knowledge about OOD samples in external datasets, unsupervised generative learning is a preferable solution which trains the model to discern the normal characteristics of in-distribution (ID) data. The hypothesis is that during inference, the model aims to reconstruct ID samples accurately, while OOD samples exhibit poorer reconstruction due to their divergence from normality. Inspired by state-of-the-art (SOTA) hybrid architectures combining CNNs and transformers, we developed a novel backbone - HAND, for detecting OOD from large-scale digital screening mammogram studies. To boost the learning efficiency, we incorporated synthetic OOD samples and a parallel discriminator in the latent space to distinguish between ID and OOD samples. Gradient reversal to the OOD reconstruction loss penalizes the model for learning OOD reconstructions. An anomaly score is computed by weighting the reconstruction and discriminator loss. On internal RSNA mammogram held-out test and external Mayo clinic hand-curated dataset, the proposed HAND model outperformed encoder-based and GAN-based baselines, and interestingly, it also outperformed the hybrid CNN+transformer baselines. Therefore, the proposed HAND pipeline offers an automated efficient computational solution for domain-specific quality checks in external screening mammograms, yielding actionable insights without direct exposure to the private medical imaging data.
Knowledge-grounded Adaptation Strategy for Vision-language Models: Building Unique Case-set for Screening Mammograms for Residents Training
May 30, 2024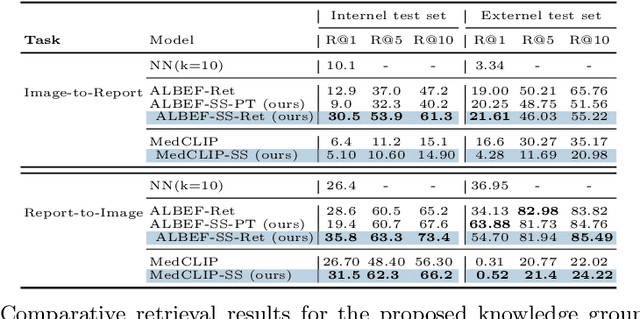
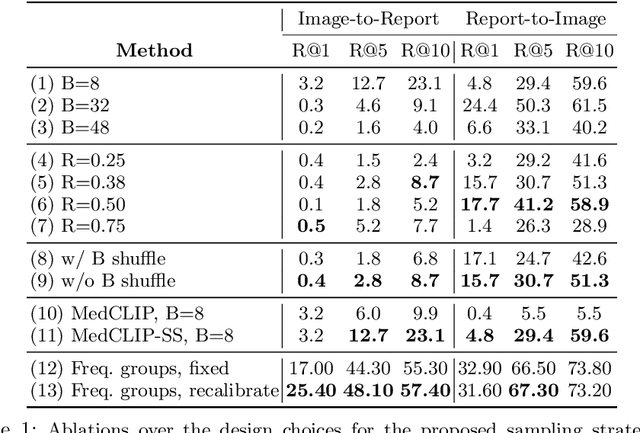
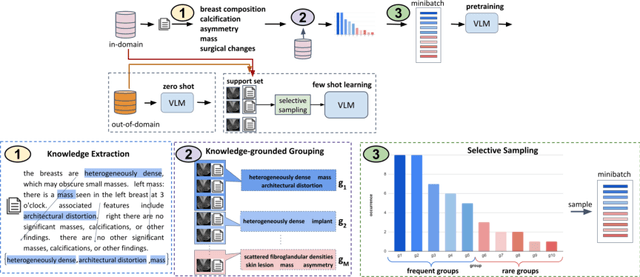
Abstract:A visual-language model (VLM) pre-trained on natural images and text pairs poses a significant barrier when applied to medical contexts due to domain shift. Yet, adapting or fine-tuning these VLMs for medical use presents considerable hurdles, including domain misalignment, limited access to extensive datasets, and high-class imbalances. Hence, there is a pressing need for strategies to effectively adapt these VLMs to the medical domain, as such adaptations would prove immensely valuable in healthcare applications. In this study, we propose a framework designed to adeptly tailor VLMs to the medical domain, employing selective sampling and hard-negative mining techniques for enhanced performance in retrieval tasks. We validate the efficacy of our proposed approach by implementing it across two distinct VLMs: the in-domain VLM (MedCLIP) and out-of-domain VLMs (ALBEF). We assess the performance of these models both in their original off-the-shelf state and after undergoing our proposed training strategies, using two extensive datasets containing mammograms and their corresponding reports. Our evaluation spans zero-shot, few-shot, and supervised scenarios. Through our approach, we observe a notable enhancement in Recall@K performance for the image-text retrieval task.
Assessing Empathy in Large Language Models with Real-World Physician-Patient Interactions
May 26, 2024Abstract:The integration of Large Language Models (LLMs) into the healthcare domain has the potential to significantly enhance patient care and support through the development of empathetic, patient-facing chatbots. This study investigates an intriguing question Can ChatGPT respond with a greater degree of empathy than those typically offered by physicians? To answer this question, we collect a de-identified dataset of patient messages and physician responses from Mayo Clinic and generate alternative replies using ChatGPT. Our analyses incorporate novel empathy ranking evaluation (EMRank) involving both automated metrics and human assessments to gauge the empathy level of responses. Our findings indicate that LLM-powered chatbots have the potential to surpass human physicians in delivering empathetic communication, suggesting a promising avenue for enhancing patient care and reducing professional burnout. The study not only highlights the importance of empathy in patient interactions but also proposes a set of effective automatic empathy ranking metrics, paving the way for the broader adoption of LLMs in healthcare.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge