Bhavik Patel
Opportunistic Screening for Pancreatic Cancer using Computed Tomography Imaging and Radiology Reports
Mar 31, 2025Abstract:Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer, with most cases diagnosed at stage IV and a five-year overall survival rate below 5%. Early detection and prognosis modeling are crucial for improving patient outcomes and guiding early intervention strategies. In this study, we developed and evaluated a deep learning fusion model that integrates radiology reports and CT imaging to predict PDAC risk. The model achieved a concordance index (C-index) of 0.6750 (95% CI: 0.6429, 0.7121) and 0.6435 (95% CI: 0.6055, 0.6789) on the internal and external dataset, respectively, for 5-year survival risk estimation. Kaplan-Meier analysis demonstrated significant separation (p<0.0001) between the low and high risk groups predicted by the fusion model. These findings highlight the potential of deep learning-based survival models in leveraging clinical and imaging data for pancreatic cancer.
Unsupervised Hybrid framework for ANomaly Detection (HAND) -- applied to Screening Mammogram
Sep 17, 2024
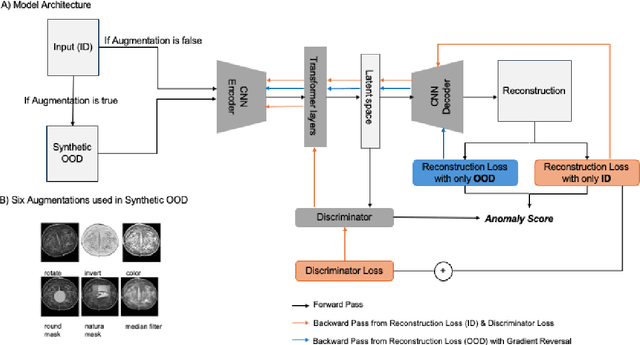
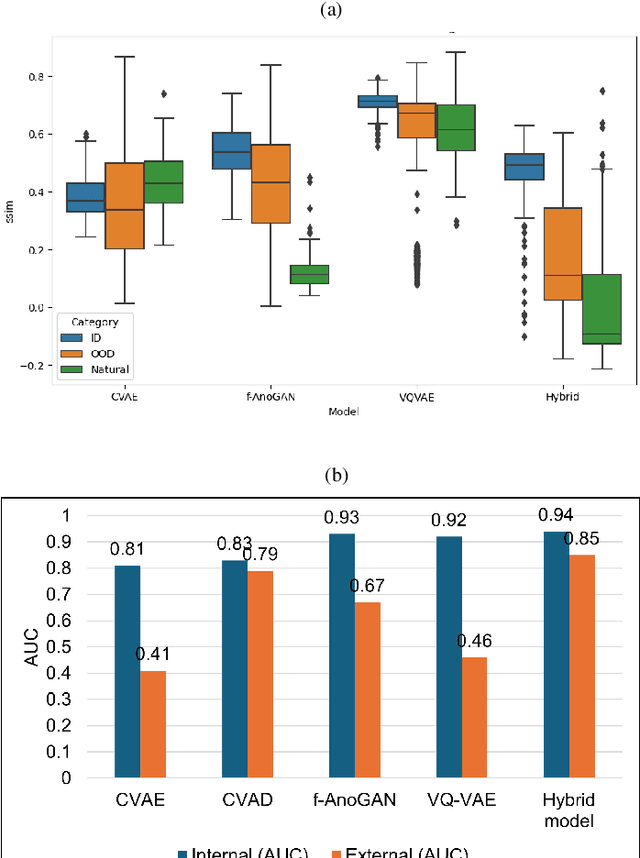
Abstract:Out-of-distribution (OOD) detection is crucial for enhancing the generalization of AI models used in mammogram screening. Given the challenge of limited prior knowledge about OOD samples in external datasets, unsupervised generative learning is a preferable solution which trains the model to discern the normal characteristics of in-distribution (ID) data. The hypothesis is that during inference, the model aims to reconstruct ID samples accurately, while OOD samples exhibit poorer reconstruction due to their divergence from normality. Inspired by state-of-the-art (SOTA) hybrid architectures combining CNNs and transformers, we developed a novel backbone - HAND, for detecting OOD from large-scale digital screening mammogram studies. To boost the learning efficiency, we incorporated synthetic OOD samples and a parallel discriminator in the latent space to distinguish between ID and OOD samples. Gradient reversal to the OOD reconstruction loss penalizes the model for learning OOD reconstructions. An anomaly score is computed by weighting the reconstruction and discriminator loss. On internal RSNA mammogram held-out test and external Mayo clinic hand-curated dataset, the proposed HAND model outperformed encoder-based and GAN-based baselines, and interestingly, it also outperformed the hybrid CNN+transformer baselines. Therefore, the proposed HAND pipeline offers an automated efficient computational solution for domain-specific quality checks in external screening mammograms, yielding actionable insights without direct exposure to the private medical imaging data.
Scout-Net: Prospective Personalized Estimation of CT Organ Doses from Scout Views
Dec 23, 2023Abstract:Purpose: Estimation of patient-specific organ doses is required for more comprehensive dose metrics, such as effective dose. Currently, available methods are performed retrospectively using the CT images themselves, which can only be done after the scan. To optimize CT acquisitions before scanning, rapid prediction of patient-specific organ dose is needed prospectively, using available scout images. We, therefore, devise an end-to-end, fully-automated deep learning solution to perform real-time, patient-specific, organ-level dosimetric estimation of CT scans. Approach: We propose the Scout-Net model for CT dose prediction at six different organs as well as for the overall patient body, leveraging the routinely obtained frontal and lateral scout images of patients, before their CT scans. To obtain reference values of the organ doses, we used Monte Carlo simulation and 3D segmentation methods on the corresponding CT images of the patients. Results: We validate our proposed Scout-Net model against real patient CT data and demonstrate the effectiveness in estimating organ doses in real-time (only 27 ms on average per scan). Additionally, we demonstrate the efficiency (real-time execution), sufficiency (reasonable error rates), and robustness (consistent across varying patient sizes) of the Scout-Net model. Conclusions: An effective, efficient, and robust Scout-Net model, once incorporated into the CT acquisition plan, could potentially guide the automatic exposure control for balanced image quality and radiation dose.
Two-step adversarial debiasing with partial learning -- medical image case-studies
Nov 16, 2021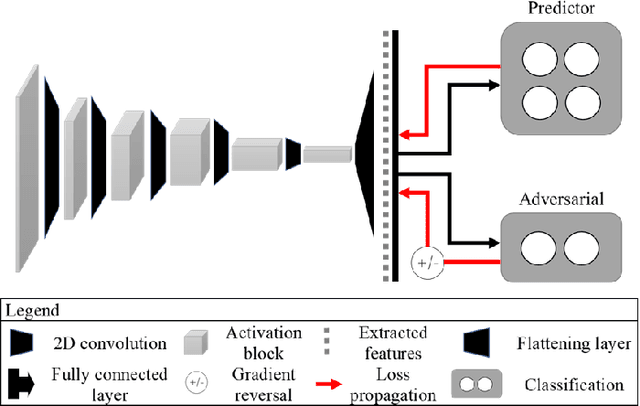
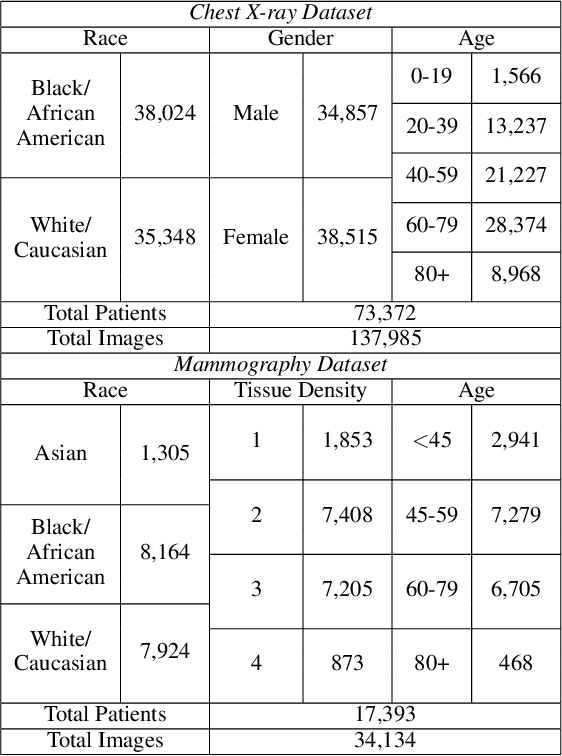
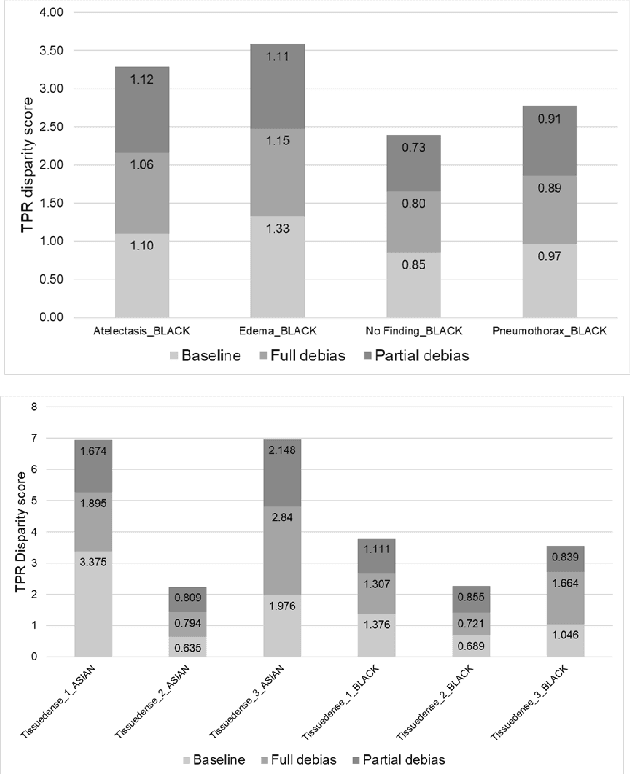
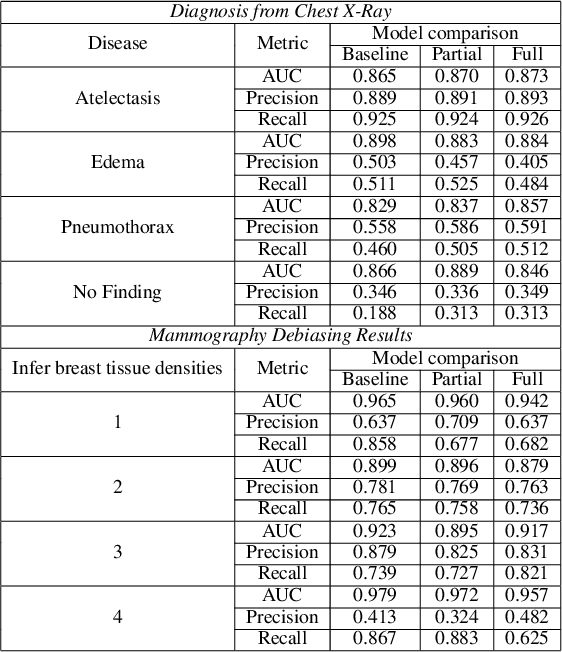
Abstract:The use of artificial intelligence (AI) in healthcare has become a very active research area in the last few years. While significant progress has been made in image classification tasks, only a few AI methods are actually being deployed in hospitals. A major hurdle in actively using clinical AI models currently is the trustworthiness of these models. More often than not, these complex models are black boxes in which promising results are generated. However, when scrutinized, these models begin to reveal implicit biases during the decision making, such as detecting race and having bias towards ethnic groups and subpopulations. In our ongoing study, we develop a two-step adversarial debiasing approach with partial learning that can reduce the racial disparity while preserving the performance of the targeted task. The methodology has been evaluated on two independent medical image case-studies - chest X-ray and mammograms, and showed promises in bias reduction while preserving the targeted performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge