Sandeep Dutta
Scout-Net: Prospective Personalized Estimation of CT Organ Doses from Scout Views
Dec 23, 2023Abstract:Purpose: Estimation of patient-specific organ doses is required for more comprehensive dose metrics, such as effective dose. Currently, available methods are performed retrospectively using the CT images themselves, which can only be done after the scan. To optimize CT acquisitions before scanning, rapid prediction of patient-specific organ dose is needed prospectively, using available scout images. We, therefore, devise an end-to-end, fully-automated deep learning solution to perform real-time, patient-specific, organ-level dosimetric estimation of CT scans. Approach: We propose the Scout-Net model for CT dose prediction at six different organs as well as for the overall patient body, leveraging the routinely obtained frontal and lateral scout images of patients, before their CT scans. To obtain reference values of the organ doses, we used Monte Carlo simulation and 3D segmentation methods on the corresponding CT images of the patients. Results: We validate our proposed Scout-Net model against real patient CT data and demonstrate the effectiveness in estimating organ doses in real-time (only 27 ms on average per scan). Additionally, we demonstrate the efficiency (real-time execution), sufficiency (reasonable error rates), and robustness (consistent across varying patient sizes) of the Scout-Net model. Conclusions: An effective, efficient, and robust Scout-Net model, once incorporated into the CT acquisition plan, could potentially guide the automatic exposure control for balanced image quality and radiation dose.
Automated Identification of Failure Cases in Organ at Risk Segmentation Using Distance Metrics: A Study on CT Data
Aug 21, 2023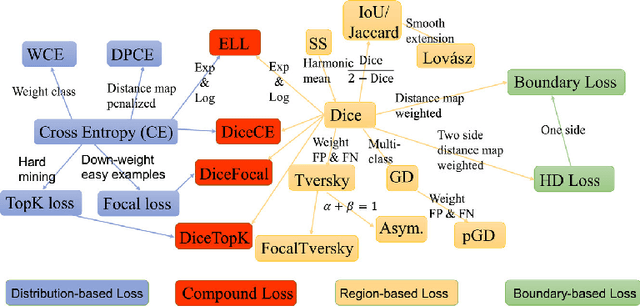
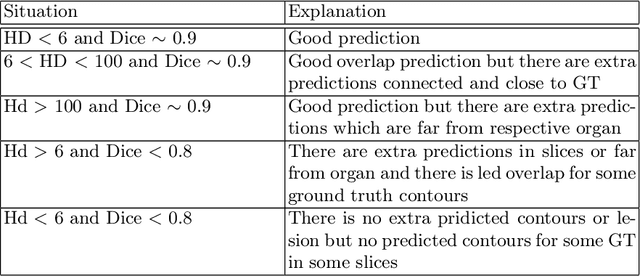
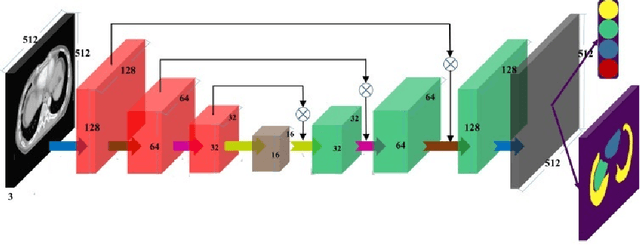
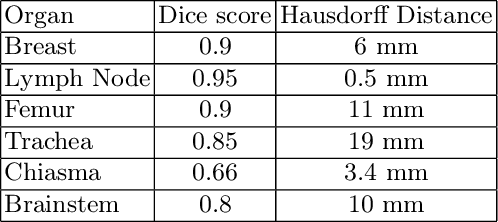
Abstract:Automated organ at risk (OAR) segmentation is crucial for radiation therapy planning in CT scans, but the generated contours by automated models can be inaccurate, potentially leading to treatment planning issues. The reasons for these inaccuracies could be varied, such as unclear organ boundaries or inaccurate ground truth due to annotation errors. To improve the model's performance, it is necessary to identify these failure cases during the training process and to correct them with some potential post-processing techniques. However, this process can be time-consuming, as traditionally it requires manual inspection of the predicted output. This paper proposes a method to automatically identify failure cases by setting a threshold for the combination of Dice and Hausdorff distances. This approach reduces the time-consuming task of visually inspecting predicted outputs, allowing for faster identification of failure case candidates. The method was evaluated on 20 cases of six different organs in CT images from clinical expert curated datasets. By setting the thresholds for the Dice and Hausdorff distances, the study was able to differentiate between various states of failure cases and evaluate over 12 cases visually. This thresholding approach could be extended to other organs, leading to faster identification of failure cases and thereby improving the quality of radiation therapy planning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge