Qicheng Lao
Voronoi-grid-based Pareto Front Learning and Its Application to Collaborative Federated Learning
May 27, 2025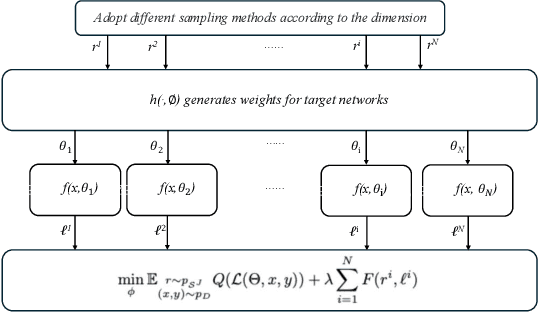
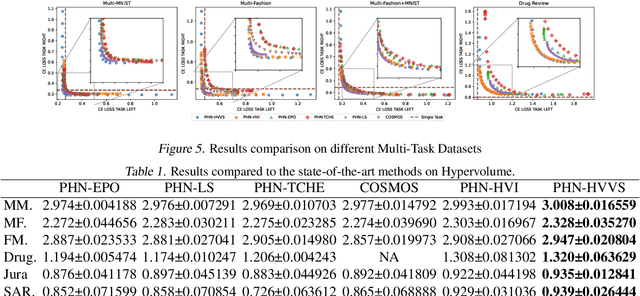
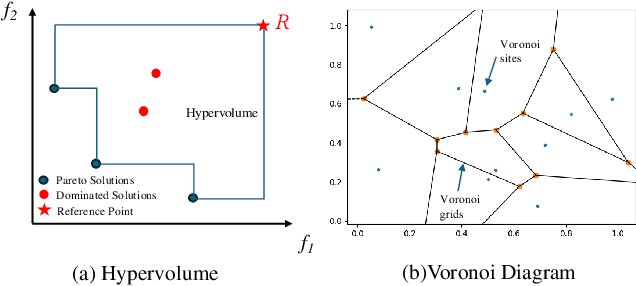
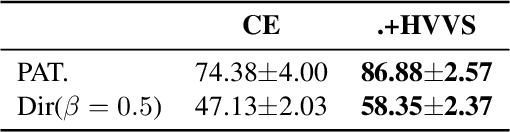
Abstract:Multi-objective optimization (MOO) exists extensively in machine learning, and aims to find a set of Pareto-optimal solutions, called the Pareto front, e.g., it is fundamental for multiple avenues of research in federated learning (FL). Pareto-Front Learning (PFL) is a powerful method implemented using Hypernetworks (PHNs) to approximate the Pareto front. This method enables the acquisition of a mapping function from a given preference vector to the solutions on the Pareto front. However, most existing PFL approaches still face two challenges: (a) sampling rays in high-dimensional spaces; (b) failing to cover the entire Pareto Front which has a convex shape. Here, we introduce a novel PFL framework, called as PHN-HVVS, which decomposes the design space into Voronoi grids and deploys a genetic algorithm (GA) for Voronoi grid partitioning within high-dimensional space. We put forward a new loss function, which effectively contributes to more extensive coverage of the resultant Pareto front and maximizes the HV Indicator. Experimental results on multiple MOO machine learning tasks demonstrate that PHN-HVVS outperforms the baselines significantly in generating Pareto front. Also, we illustrate that PHN-HVVS advances the methodologies of several recent problems in the FL field. The code is available at https://github.com/buptcmm/phnhvvs}{https://github.com/buptcmm/phnhvvs.
One-to-Normal: Anomaly Personalization for Few-shot Anomaly Detection
Feb 03, 2025



Abstract:Traditional Anomaly Detection (AD) methods have predominantly relied on unsupervised learning from extensive normal data. Recent AD methods have evolved with the advent of large pre-trained vision-language models, enhancing few-shot anomaly detection capabilities. However, these latest AD methods still exhibit limitations in accuracy improvement. One contributing factor is their direct comparison of a query image's features with those of few-shot normal images. This direct comparison often leads to a loss of precision and complicates the extension of these techniques to more complex domains--an area that remains underexplored in a more refined and comprehensive manner. To address these limitations, we introduce the anomaly personalization method, which performs a personalized one-to-normal transformation of query images using an anomaly-free customized generation model, ensuring close alignment with the normal manifold. Moreover, to further enhance the stability and robustness of prediction results, we propose a triplet contrastive anomaly inference strategy, which incorporates a comprehensive comparison between the query and generated anomaly-free data pool and prompt information. Extensive evaluations across eleven datasets in three domains demonstrate our model's effectiveness compared to the latest AD methods. Additionally, our method has been proven to transfer flexibly to other AD methods, with the generated image data effectively improving the performance of other AD methods.
Guiding Medical Vision-Language Models with Explicit Visual Prompts: Framework Design and Comprehensive Exploration of Prompt Variations
Jan 04, 2025



Abstract:With the recent advancements in vision-language models (VLMs) driven by large language models (LLMs), many researchers have focused on models that comprised of an image encoder, an image-to-language projection layer, and a text decoder architectures, leading to the emergence of works like LLava-Med. However, these works primarily operate at the whole-image level, aligning general information from 2D medical images without attending to finer details. As a result, these models often provide irrelevant or non-clinically valuable information while missing critical details. Medical vision-language tasks differ significantly from general images, particularly in their focus on fine-grained details, while excluding irrelevant content. General domain VLMs tend to prioritize global information due to their design, which compresses the entire image into a multi-token representation that is passed into the LLM decoder. Therefore, current VLMs all lack the capability to restrict their attention to particular areas. To address this critical issue in the medical domain, we introduce MedVP, an visual prompt generation and fine-tuning framework, which involves extract medical entities, generate visual prompts, and adapt datasets for visual prompt guided fine-tuning. To the best of our knowledge, this is the first work to explicitly introduce visual prompt into medical VLMs, and we successfully outperform recent state-of-the-art large models across multiple medical VQA datasets. Extensive experiments are conducted to analyze the impact of different visual prompt forms and how they contribute to performance improvement. The results demonstrate both the effectiveness and clinical significance of our approach
Evaluating Hallucination in Text-to-Image Diffusion Models with Scene-Graph based Question-Answering Agent
Dec 07, 2024



Abstract:Contemporary Text-to-Image (T2I) models frequently depend on qualitative human evaluations to assess the consistency between synthesized images and the text prompts. There is a demand for quantitative and automatic evaluation tools, given that human evaluation lacks reproducibility. We believe that an effective T2I evaluation metric should accomplish the following: detect instances where the generated images do not align with the textual prompts, a discrepancy we define as the `hallucination problem' in T2I tasks; record the types and frequency of hallucination issues, aiding users in understanding the causes of errors; and provide a comprehensive and intuitive scoring that close to human standard. To achieve these objectives, we propose a method based on large language models (LLMs) for conducting question-answering with an extracted scene-graph and created a dataset with human-rated scores for generated images. From the methodology perspective, we combine knowledge-enhanced question-answering tasks with image evaluation tasks, making the evaluation metrics more controllable and easier to interpret. For the contribution on the dataset side, we generated 12,000 synthesized images based on 1,000 composited prompts using three advanced T2I models. Subsequently, we conduct human scoring on all synthesized images and prompt pairs to validate the accuracy and effectiveness of our method as an evaluation metric. All generated images and the human-labeled scores will be made publicly available in the future to facilitate ongoing research on this crucial issue. Extensive experiments show that our method aligns more closely with human scoring patterns than other evaluation metrics.
Free-Rider and Conflict Aware Collaboration Formation for Cross-Silo Federated Learning
Oct 28, 2024



Abstract:Federated learning (FL) is a machine learning paradigm that allows multiple FL participants (FL-PTs) to collaborate on training models without sharing private data. Due to data heterogeneity, negative transfer may occur in the FL training process. This necessitates FL-PT selection based on their data complementarity. In cross-silo FL, organizations that engage in business activities are key sources of FL-PTs. The resulting FL ecosystem has two features: (i) self-interest, and (ii) competition among FL-PTs. This requires the desirable FL-PT selection strategy to simultaneously mitigate the problems of free riders and conflicts of interest among competitors. To this end, we propose an optimal FL collaboration formation strategy -- FedEgoists -- which ensures that: (1) a FL-PT can benefit from FL if and only if it benefits the FL ecosystem, and (2) a FL-PT will not contribute to its competitors or their supporters. It provides an efficient clustering solution to group FL-PTs into coalitions, ensuring that within each coalition, FL-PTs share the same interest. We theoretically prove that the FL-PT coalitions formed are optimal since no coalitions can collaborate together to improve the utility of any of their members. Extensive experiments on widely adopted benchmark datasets demonstrate the effectiveness of FedEgoists compared to nine state-of-the-art baseline methods, and its ability to establish efficient collaborative networks in cross-silos FL with FL-PTs that engage in business activities.
Benchmarking Data Heterogeneity Evaluation Approaches for Personalized Federated Learning
Oct 09, 2024



Abstract:There is growing research interest in measuring the statistical heterogeneity of clients' local datasets. Such measurements are used to estimate the suitability for collaborative training of personalized federated learning (PFL) models. Currently, these research endeavors are taking place in silos and there is a lack of a unified benchmark to provide a fair and convenient comparison among various approaches in common settings. We aim to bridge this important gap in this paper. The proposed benchmarking framework currently includes six representative approaches. Extensive experiments have been conducted to compare these approaches under five standard non-IID FL settings, providing much needed insights into which approaches are advantageous under which settings. The proposed framework offers useful guidance on the suitability of various data divergence measures in FL systems. It is beneficial for keeping related research activities on the right track in terms of: (1) designing PFL schemes, (2) selecting appropriate data heterogeneity evaluation approaches for specific FL application scenarios, and (3) addressing fairness issues in collaborative model training. The code is available at https://github.com/Xiaoni-61/DH-Benchmark.
Curriculum Prompting Foundation Models for Medical Image Segmentation
Sep 01, 2024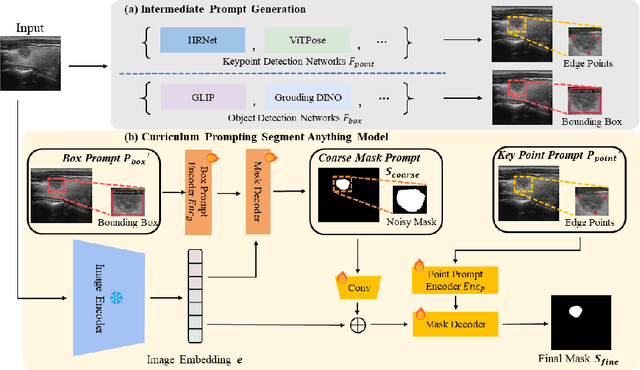
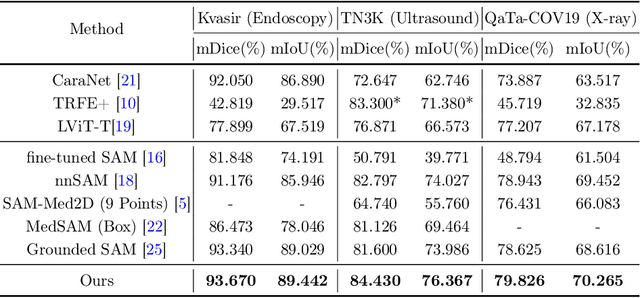
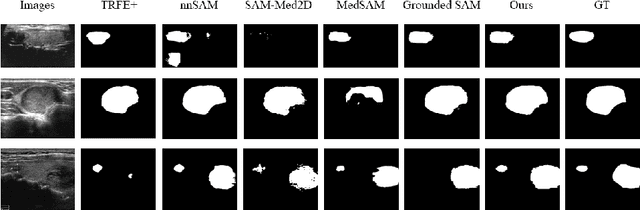
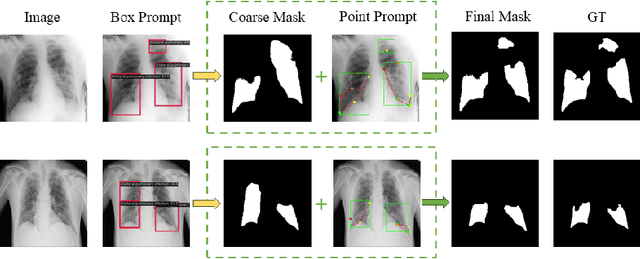
Abstract:Adapting large pre-trained foundation models, e.g., SAM, for medical image segmentation remains a significant challenge. A crucial step involves the formulation of a series of specialized prompts that incorporate specific clinical instructions. Past works have been heavily reliant on a singular type of prompt for each instance, necessitating manual input of an ideally correct prompt, which is less efficient. To tackle this issue, we propose to utilize prompts of different granularity, which are sourced from original images to provide a broader scope of clinical insights. However, combining prompts of varying types can pose a challenge due to potential conflicts. In response, we have designed a coarse-to-fine mechanism, referred to as curriculum prompting, that progressively integrates prompts of different types. Through extensive experiments on three public medical datasets across various modalities, we demonstrate the effectiveness of our proposed approach, which not only automates the prompt generation process but also yields superior performance compared to other SAM-based medical image segmentation methods. Code is available at: https://github.com/AnnaZzz-zxq/Curriculum-Prompting.
MLAE: Masked LoRA Experts for Parameter-Efficient Fine-Tuning
May 29, 2024



Abstract:In response to the challenges posed by the extensive parameter updates required for full fine-tuning of large-scale pre-trained models, parameter-efficient fine-tuning (PEFT) methods, exemplified by Low-Rank Adaptation (LoRA), have emerged. LoRA simplifies the fine-tuning process but may still struggle with a certain level of redundancy in low-rank matrices and limited effectiveness from merely increasing their rank. To address these issues, a natural idea is to enhance the independence and diversity of the learning process for the low-rank matrices. Therefore, we propose Masked LoRA Experts (MLAE), an innovative approach that applies the concept of masking to PEFT. Our method incorporates a cellular decomposition strategy that transforms a low-rank matrix into independent rank-1 submatrices, or ``experts'', thus enhancing independence. Additionally, we introduce a binary mask matrix that selectively activates these experts during training to promote more diverse and anisotropic learning, based on expert-level dropout strategies. Our investigations reveal that this selective activation not only enhances performance but also fosters a more diverse acquisition of knowledge with a marked decrease in parameter similarity among MLAE, significantly boosting the quality of the model while barely increasing the parameter count. Remarkably, MLAE achieves new SOTA performance with an average accuracy score of 78.8% on the VTAB-1k benchmark and 90.9% on the FGVC benchmark, demonstrating superior performance. Our code is available at https://github.com/jie040109/MLAE.
OpenMEDLab: An Open-source Platform for Multi-modality Foundation Models in Medicine
Mar 04, 2024



Abstract:The emerging trend of advancing generalist artificial intelligence, such as GPTv4 and Gemini, has reshaped the landscape of research (academia and industry) in machine learning and many other research areas. However, domain-specific applications of such foundation models (e.g., in medicine) remain untouched or often at their very early stages. It will require an individual set of transfer learning and model adaptation techniques by further expanding and injecting these models with domain knowledge and data. The development of such technologies could be largely accelerated if the bundle of data, algorithms, and pre-trained foundation models were gathered together and open-sourced in an organized manner. In this work, we present OpenMEDLab, an open-source platform for multi-modality foundation models. It encapsulates not only solutions of pioneering attempts in prompting and fine-tuning large language and vision models for frontline clinical and bioinformatic applications but also building domain-specific foundation models with large-scale multi-modal medical data. Importantly, it opens access to a group of pre-trained foundation models for various medical image modalities, clinical text, protein engineering, etc. Inspiring and competitive results are also demonstrated for each collected approach and model in a variety of benchmarks for downstream tasks. We welcome researchers in the field of medical artificial intelligence to continuously contribute cutting-edge methods and models to OpenMEDLab, which can be accessed via https://github.com/openmedlab.
Increasing SAM Zero-Shot Performance on Multimodal Medical Images Using GPT-4 Generated Descriptive Prompts Without Human Annotation
Feb 24, 2024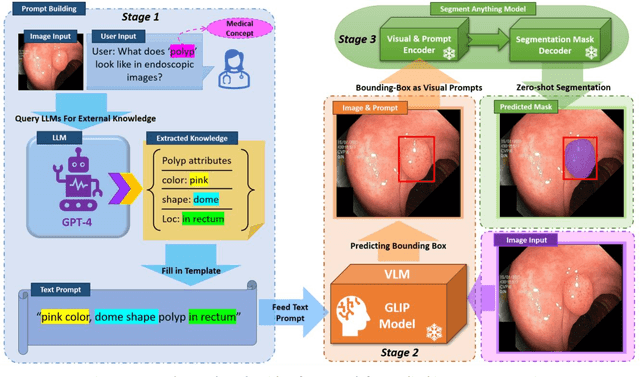
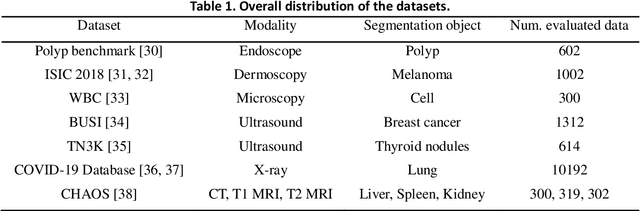
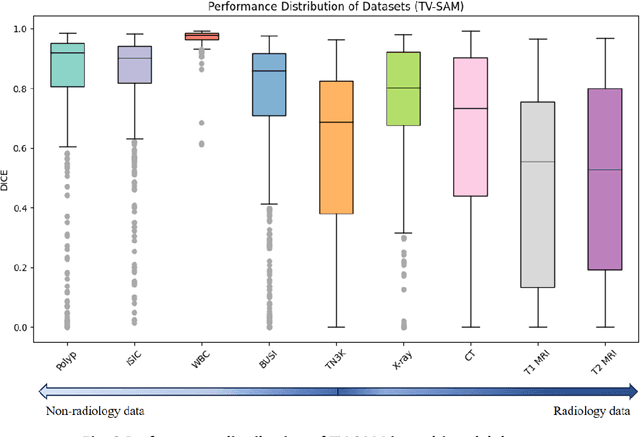
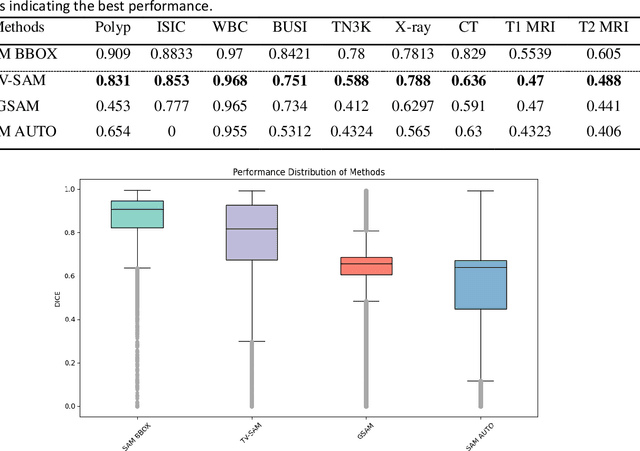
Abstract:This study develops and evaluates a novel multimodal medical image zero-shot segmentation algorithm named Text-Visual-Prompt SAM (TV-SAM) without any manual annotations. TV-SAM incorporates and integrates large language model GPT-4, Vision Language Model GLIP, and Segment Anything Model (SAM), to autonomously generate descriptive text prompts and visual bounding box prompts from medical images, thereby enhancing SAM for zero-shot segmentation. Comprehensive evaluations are implemented on seven public datasets encompassing eight imaging modalities to demonstrate that TV-SAM can effectively segment unseen targets across various modalities without additional training, significantly outperforming SAM AUTO and GSAM, closely matching the performance of SAM BBOX with gold standard bounding box prompts, and surpassing the state-of-the-art on specific datasets like ISIC and WBC. The study indicates that TV-SAM serves as an effective multimodal medical image zero-shot segmentation algorithm, highlighting the significant contribution of GPT-4 to zero-shot segmentation. By integrating foundational models such as GPT-4, GLIP, and SAM, it could enhance the capability to address complex problems in specialized domains. The code is available at: https://github.com/JZK00/TV-SAM.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge