Yogesh Rathi
Cross-Population White Matter Atlas Creation for Concurrent Mapping of Brain Connections in Neonates and Adults with Diffusion MRI Tractography
Dec 23, 2025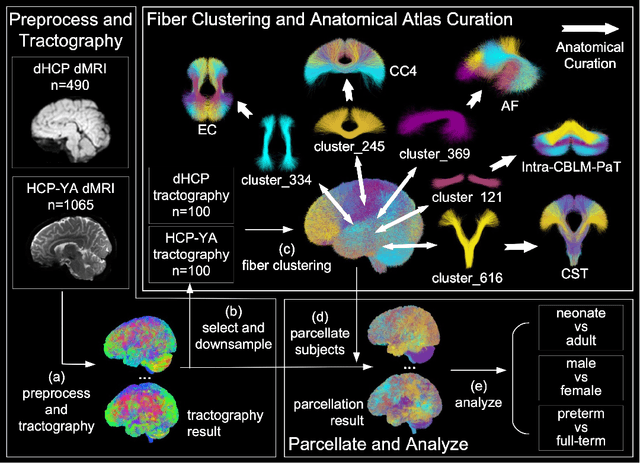
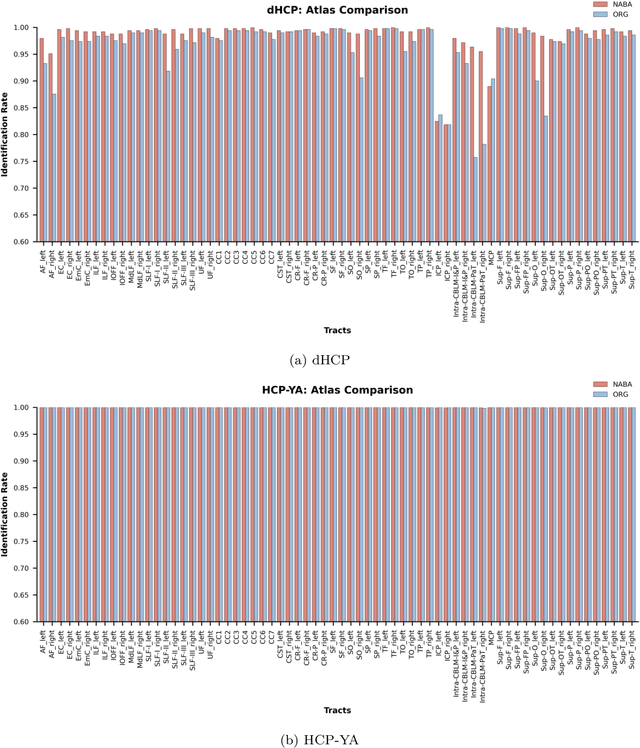
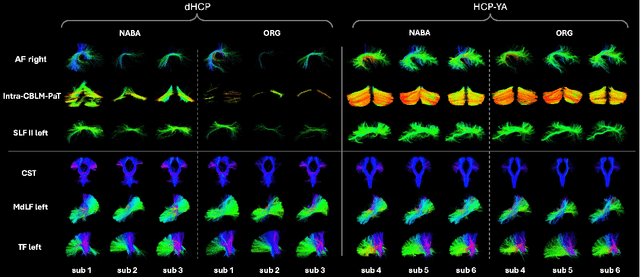
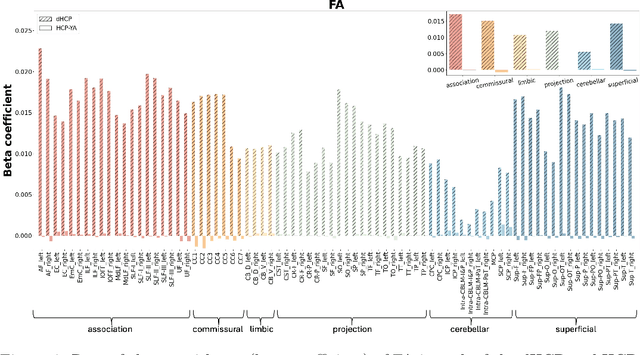
Abstract:Comparing white matter (WM) connections between adults and neonates using diffusion MRI (dMRI) can advance our understanding of typical brain development and potential biomarkers for neurological disorders. However, existing WM atlases are population-specific (adult or neonatal) and reside in separate spaces, preventing direct cross-population comparisons. A unified WM atlas spanning both neonates and adults is still lacking. In this study, we propose a neonatal/adult brain atlas (NABA), a WM tractography atlas built from dMRI data of both neonates and adults. NABA is constructed using a robust, data-driven fiber clustering pipeline, enabling group-wise WM atlasing across populations despite substantial anatomical variability. The atlas provides a standardized template for WM parcellation, allowing direct comparison of WM tracts between neonates and adults. Using NABA, we conduct four analyses: (1) evaluating the feasibility of joint WM mapping across populations, (2) characterizing WM development across neonatal ages relative to adults, (3) assessing sex-related differences in neonatal WM development, and (4) examining the effects of preterm birth. Our results show that NABA robustly identifies WM tracts in both populations. We observe rapid fractional anisotropy (FA) development in long-range association tracts, including the arcuate fasciculus and superior longitudinal fasciculus II, whereas intra-cerebellar tracts develop more slowly. Neonatal females exhibit faster overall FA development than males. Although preterm neonates show lower overall FA development rates, they demonstrate relatively higher FA growth in specific tracts, including the corticospinal tract, corona radiata-pontine pathway, and intracerebellar tracts. These findings demonstrate that NABA is a useful tool for investigating WM development across neonates and adults.
DDTracking: A Deep Generative Framework for Diffusion MRI Tractography with Streamline Local-Global Spatiotemporal Modeling
Aug 06, 2025Abstract:This paper presents DDTracking, a novel deep generative framework for diffusion MRI tractography that formulates streamline propagation as a conditional denoising diffusion process. In DDTracking, we introduce a dual-pathway encoding network that jointly models local spatial encoding (capturing fine-scale structural details at each streamline point) and global temporal dependencies (ensuring long-range consistency across the entire streamline). Furthermore, we design a conditional diffusion model module, which leverages the learned local and global embeddings to predict streamline propagation orientations for tractography in an end-to-end trainable manner. We conduct a comprehensive evaluation across diverse, independently acquired dMRI datasets, including both synthetic and clinical data. Experiments on two well-established benchmarks with ground truth (ISMRM Challenge and TractoInferno) demonstrate that DDTracking largely outperforms current state-of-the-art tractography methods. Furthermore, our results highlight DDTracking's strong generalizability across heterogeneous datasets, spanning varying health conditions, age groups, imaging protocols, and scanner types. Collectively, DDTracking offers anatomically plausible and robust tractography, presenting a scalable, adaptable, and end-to-end learnable solution for broad dMRI applications. Code is available at: https://github.com/yishengpoxiao/DDtracking.git
A Multimodal Deep Learning Approach for White Matter Shape Prediction in Diffusion MRI Tractography
Apr 25, 2025Abstract:Shape measures have emerged as promising descriptors of white matter tractography, offering complementary insights into anatomical variability and associations with cognitive and clinical phenotypes. However, conventional methods for computing shape measures are computationally expensive and time-consuming for large-scale datasets due to reliance on voxel-based representations. We propose Tract2Shape, a novel multimodal deep learning framework that leverages geometric (point cloud) and scalar (tabular) features to predict ten white matter tractography shape measures. To enhance model efficiency, we utilize a dimensionality reduction algorithm for the model to predict five primary shape components. The model is trained and evaluated on two independently acquired datasets, the HCP-YA dataset, and the PPMI dataset. We evaluate the performance of Tract2Shape by training and testing it on the HCP-YA dataset and comparing the results with state-of-the-art models. To further assess its robustness and generalization ability, we also test Tract2Shape on the unseen PPMI dataset. Tract2Shape outperforms SOTA deep learning models across all ten shape measures, achieving the highest average Pearson's r and the lowest nMSE on the HCP-YA dataset. The ablation study shows that both multimodal input and PCA contribute to performance gains. On the unseen testing PPMI dataset, Tract2Shape maintains a high Pearson's r and low nMSE, demonstrating strong generalizability in cross-dataset evaluation. Tract2Shape enables fast, accurate, and generalizable prediction of white matter shape measures from tractography data, supporting scalable analysis across datasets. This framework lays a promising foundation for future large-scale white matter shape analysis.
DeepNuParc: A Novel Deep Clustering Framework for Fine-scale Parcellation of Brain Nuclei Using Diffusion MRI Tractography
Mar 10, 2025



Abstract:Brain nuclei are clusters of anatomically distinct neurons that serve as important hubs for processing and relaying information in various neural circuits. Fine-scale parcellation of the brain nuclei is vital for a comprehensive understanding of its anatomico-functional correlations. Diffusion MRI tractography is an advanced imaging technique that can estimate the brain's white matter structural connectivity to potentially reveal the topography of the nuclei of interest for studying its subdivisions. In this work, we present a deep clustering pipeline, namely DeepNuParc, to perform automated, fine-scale parcellation of brain nuclei using diffusion MRI tractography. First, we incorporate a newly proposed deep learning approach to enable accurate segmentation of the nuclei of interest directly on the dMRI data. Next, we design a novel streamline clustering-based structural connectivity feature for a robust representation of voxels within the nuclei. Finally, we improve the popular joint dimensionality reduction and k-means clustering approach to enable nuclei parcellation at a finer scale. We demonstrate DeepNuParc on two important brain structures, i.e. the amygdala and the thalamus, that are known to have multiple anatomically and functionally distinct nuclei subdivisions. Experimental results show that DeepNuParc enables consistent parcellation of the nuclei into multiple parcels across multiple subjects and achieves good correspondence with the widely used coarse-scale atlases. Our codes are available at https://github.com/HarlandZZC/deep_nuclei_parcellation.
TractCloud-FOV: Deep Learning-based Robust Tractography Parcellation in Diffusion MRI with Incomplete Field of View
Feb 28, 2025Abstract:Tractography parcellation classifies streamlines reconstructed from diffusion MRI into anatomically defined fiber tracts for clinical and research applications. However, clinical scans often have incomplete fields of view (FOV) where brain regions are partially imaged, leading to partial or truncated fiber tracts. To address this challenge, we introduce TractCloud-FOV, a deep learning framework that robustly parcellates tractography under conditions of incomplete FOV. We propose a novel training strategy, FOV-Cut Augmentation (FOV-CA), in which we synthetically cut tractograms to simulate a spectrum of real-world inferior FOV cutoff scenarios. This data augmentation approach enriches the training set with realistic truncated streamlines, enabling the model to achieve superior generalization. We evaluate the proposed TractCloud-FOV on both synthetically cut tractography and two real-life datasets with incomplete FOV. TractCloud-FOV significantly outperforms several state-of-the-art methods on all testing datasets in terms of streamline classification accuracy, generalization ability, tract anatomical depiction, and computational efficiency. Overall, TractCloud-FOV achieves efficient and consistent tractography parcellation in diffusion MRI with incomplete FOV.
Rapid Whole Brain Mesoscale In-vivo MR Imaging using Multi-scale Implicit Neural Representation
Feb 12, 2025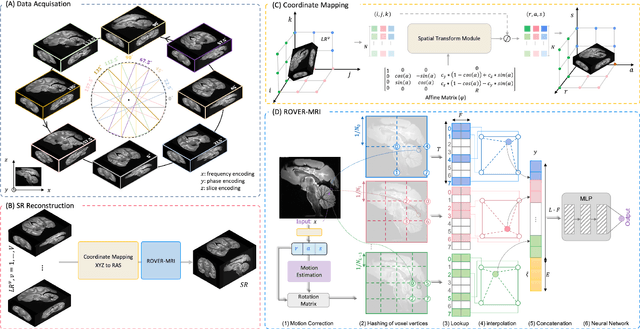
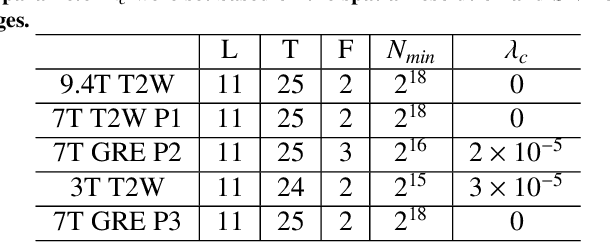
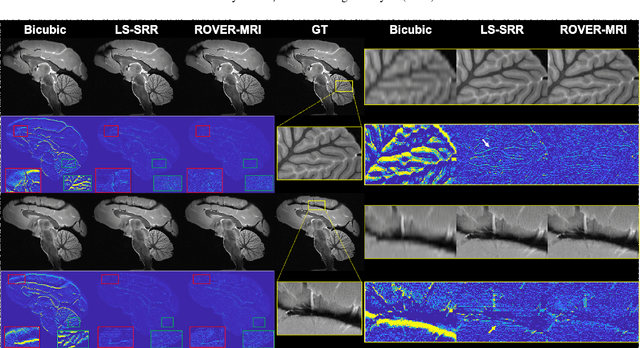

Abstract:Purpose: To develop and validate a novel image reconstruction technique using implicit neural representations (INR) for multi-view thick-slice acquisitions while reducing the scan time but maintaining high signal-to-noise ratio (SNR). Methods: We propose Rotating-view super-resolution (ROVER)-MRI, an unsupervised neural network-based algorithm designed to reconstruct MRI data from multi-view thick slices, effectively reducing scan time by 2-fold while maintaining fine anatomical details. We compare our method to both bicubic interpolation and the current state-of-the-art regularized least-squares super-resolution reconstruction (LS-SRR) technique. Validation is performed using ground-truth ex-vivo monkey brain data, and we demonstrate superior reconstruction quality across several in-vivo human datasets. Notably, we achieve the reconstruction of a whole human brain in-vivo T2-weighted image with an unprecedented 180{\mu}m isotropic spatial resolution, accomplished in just 17 minutes of scan time on a 7T MRI scanner. Results: ROVER-MRI outperformed LS-SRR method in terms of reconstruction quality with 22.4% lower relative error (RE) and 7.5% lower full-width half maximum (FWHM) indicating better preservation of fine structural details in nearly half the scan time. Conclusion: ROVER-MRI offers an efficient and robust approach for mesoscale MR imaging, enabling rapid, high-resolution whole-brain scans. Its versatility holds great promise for research applications requiring anatomical details and time-efficient imaging.
MICCAI-CDMRI 2023 QuantConn Challenge Findings on Achieving Robust Quantitative Connectivity through Harmonized Preprocessing of Diffusion MRI
Nov 14, 2024



Abstract:White matter alterations are increasingly implicated in neurological diseases and their progression. International-scale studies use diffusion-weighted magnetic resonance imaging (DW-MRI) to qualitatively identify changes in white matter microstructure and connectivity. Yet, quantitative analysis of DW-MRI data is hindered by inconsistencies stemming from varying acquisition protocols. There is a pressing need to harmonize the preprocessing of DW-MRI datasets to ensure the derivation of robust quantitative diffusion metrics across acquisitions. In the MICCAI-CDMRI 2023 QuantConn challenge, participants were provided raw data from the same individuals collected on the same scanner but with two different acquisitions and tasked with preprocessing the DW-MRI to minimize acquisition differences while retaining biological variation. Submissions are evaluated on the reproducibility and comparability of cross-acquisition bundle-wise microstructure measures, bundle shape features, and connectomics. The key innovations of the QuantConn challenge are that (1) we assess bundles and tractography in the context of harmonization for the first time, (2) we assess connectomics in the context of harmonization for the first time, and (3) we have 10x additional subjects over prior harmonization challenge, MUSHAC and 100x over SuperMUDI. We find that bundle surface area, fractional anisotropy, connectome assortativity, betweenness centrality, edge count, modularity, nodal strength, and participation coefficient measures are most biased by acquisition and that machine learning voxel-wise correction, RISH mapping, and NeSH methods effectively reduce these biases. In addition, microstructure measures AD, MD, RD, bundle length, connectome density, efficiency, and path length are least biased by these acquisition differences.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2024/019
A Novel Deep Learning Tractography Fiber Clustering Framework for Functionally Consistent White Matter Parcellation Using Multimodal Diffusion MRI and Functional MRI
Nov 04, 2024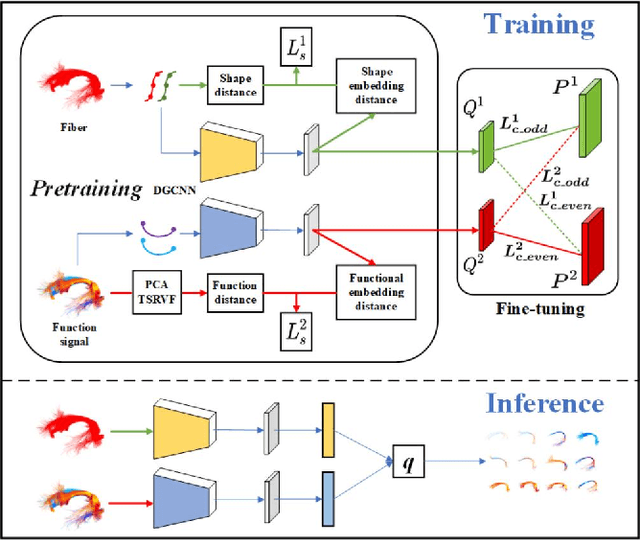
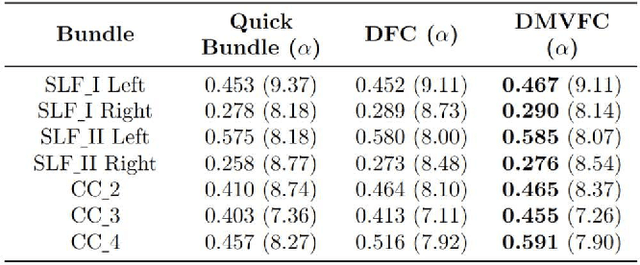
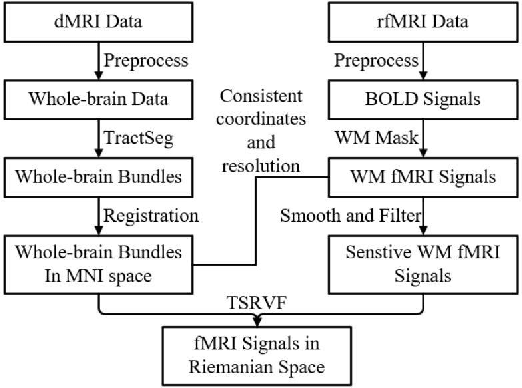
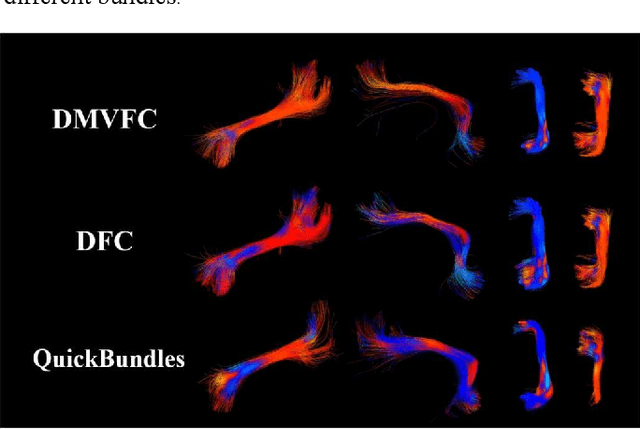
Abstract:Tractography fiber clustering using diffusion MRI (dMRI) is a crucial strategy for white matter (WM) parcellation. Current methods primarily use the geometric information of fibers (i.e., the spatial trajectories) to group similar fibers into clusters, overlooking the important functional signals present along the fiber tracts. There is increasing evidence that neural activity in the WM can be measured using functional MRI (fMRI), offering potentially valuable multimodal information for fiber clustering. In this paper, we develop a novel deep learning fiber clustering framework, namely Deep Multi-view Fiber Clustering (DMVFC), that uses joint dMRI and fMRI data to enable functionally consistent WM parcellation. DMVFC can effectively integrate the geometric characteristics of the WM fibers with the fMRI BOLD signals along the fiber tracts. It includes two major components: 1) a multi-view pretraining module to compute embedding features from fiber geometric information and functional signals separately, and 2) a collaborative fine-tuning module to simultaneously refine the two kinds of embeddings. In the experiments, we compare DMVFC with two state-of-the-art fiber clustering methods and demonstrate superior performance in achieving functionally meaningful and consistent WM parcellation results.
TractShapeNet: Efficient Multi-Shape Learning with 3D Tractography Point Clouds
Oct 29, 2024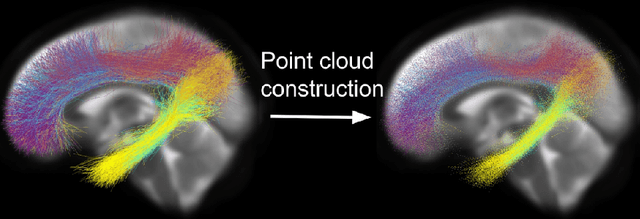
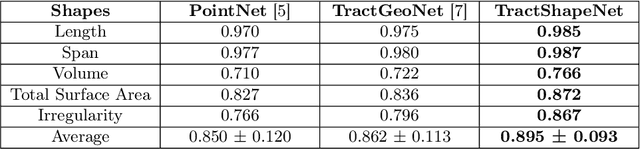

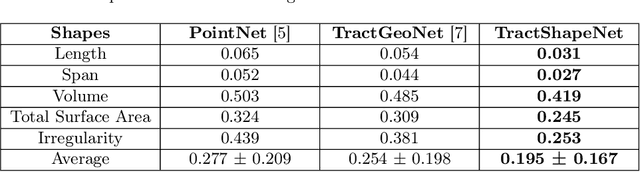
Abstract:Brain imaging studies have demonstrated that diffusion MRI tractography geometric shape descriptors can inform the study of the brain's white matter pathways and their relationship to brain function. In this work, we investigate the possibility of utilizing a deep learning model to compute shape measures of the brain's white matter connections. We introduce a novel framework, TractShapeNet, that leverages a point cloud representation of tractography to compute five shape measures: length, span, volume, total surface area, and irregularity. We assess the performance of the method on a large dataset including 1065 healthy young adults. Experiments for shape measure computation demonstrate that our proposed TractShapeNet outperforms other point cloud-based neural network models in both the Pearson correlation coefficient and normalized error metrics. We compare the inference runtime results with the conventional shape computation tool DSI-Studio. Our results demonstrate that a deep learning approach enables faster and more efficient shape measure computation. We also conduct experiments on two downstream language cognition prediction tasks, showing that shape measures from TractShapeNet perform similarly to those computed by DSI-Studio. Our code will be available at: https://github.com/SlicerDMRI/TractShapeNet.
The shape of the brain's connections is predictive of cognitive performance: an explainable machine learning study
Oct 19, 2024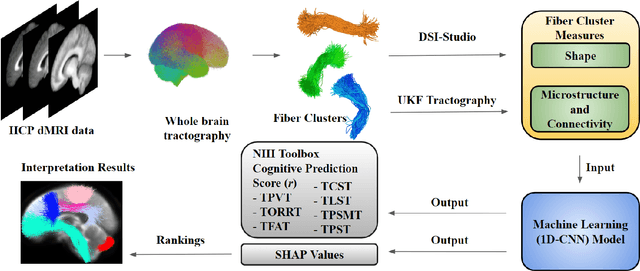
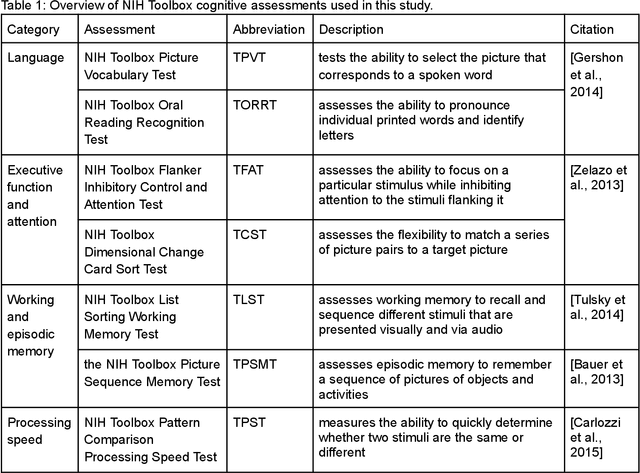

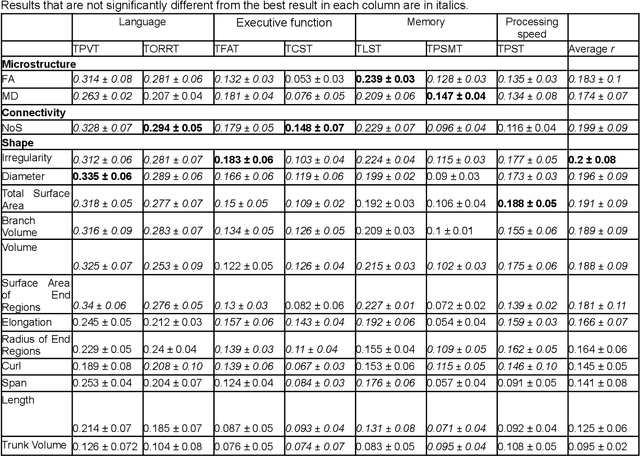
Abstract:The shape of the brain's white matter connections is relatively unexplored in diffusion MRI tractography analysis. While it is known that tract shape varies in populations and across the human lifespan, it is unknown if the variability in dMRI tractography-derived shape may relate to the brain's functional variability across individuals. This work explores the potential of leveraging tractography fiber cluster shape measures to predict subject-specific cognitive performance. We implement machine learning models to predict individual cognitive performance scores. We study a large-scale database from the HCP-YA study. We apply an atlas-based fiber cluster parcellation to the dMRI tractography of each individual. We compute 15 shape, microstructure, and connectivity features for each fiber cluster. Using these features as input, we train a total of 210 models to predict 7 different NIH Toolbox cognitive performance assessments. We apply an explainable AI technique, SHAP, to assess the importance of each fiber cluster for prediction. Our results demonstrate that shape measures are predictive of individual cognitive performance. The studied shape measures, such as irregularity, diameter, total surface area, volume, and branch volume, are as effective for prediction as microstructure and connectivity measures. The overall best-performing feature is a shape feature, irregularity, which describes how different a cluster's shape is from an idealized cylinder. Further interpretation using SHAP values suggest that fiber clusters with features highly predictive of cognitive ability are widespread throughout the brain, including fiber clusters from the superficial association, deep association, cerebellar, striatal, and projection pathways. This study demonstrates the strong potential of shape descriptors to enhance the study of the brain's white matter and its relationship to cognitive function.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge