Lipeng Ning
Rapid Whole Brain Mesoscale In-vivo MR Imaging using Multi-scale Implicit Neural Representation
Feb 12, 2025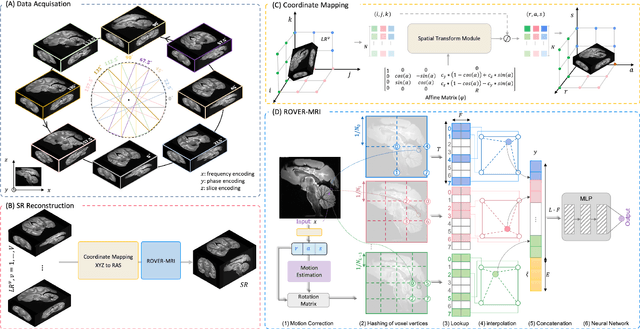
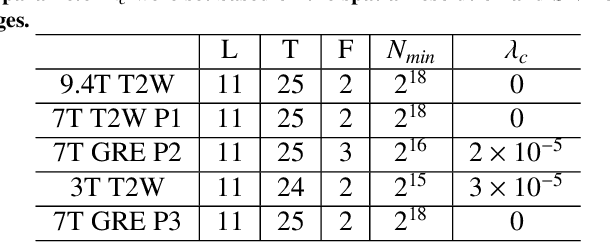
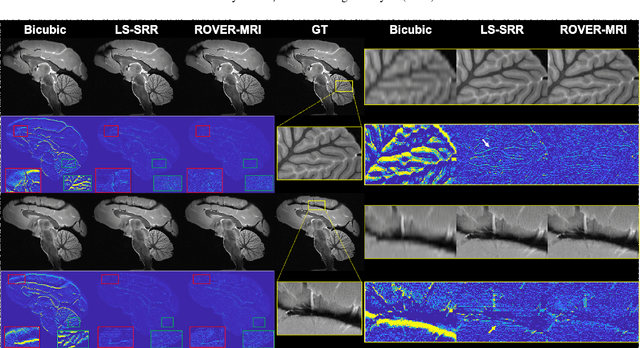

Abstract:Purpose: To develop and validate a novel image reconstruction technique using implicit neural representations (INR) for multi-view thick-slice acquisitions while reducing the scan time but maintaining high signal-to-noise ratio (SNR). Methods: We propose Rotating-view super-resolution (ROVER)-MRI, an unsupervised neural network-based algorithm designed to reconstruct MRI data from multi-view thick slices, effectively reducing scan time by 2-fold while maintaining fine anatomical details. We compare our method to both bicubic interpolation and the current state-of-the-art regularized least-squares super-resolution reconstruction (LS-SRR) technique. Validation is performed using ground-truth ex-vivo monkey brain data, and we demonstrate superior reconstruction quality across several in-vivo human datasets. Notably, we achieve the reconstruction of a whole human brain in-vivo T2-weighted image with an unprecedented 180{\mu}m isotropic spatial resolution, accomplished in just 17 minutes of scan time on a 7T MRI scanner. Results: ROVER-MRI outperformed LS-SRR method in terms of reconstruction quality with 22.4% lower relative error (RE) and 7.5% lower full-width half maximum (FWHM) indicating better preservation of fine structural details in nearly half the scan time. Conclusion: ROVER-MRI offers an efficient and robust approach for mesoscale MR imaging, enabling rapid, high-resolution whole-brain scans. Its versatility holds great promise for research applications requiring anatomical details and time-efficient imaging.
PRIME: Phase Reversed Interleaved Multi-Echo acquisition enables highly accelerated distortion-free diffusion MRI
Sep 11, 2024


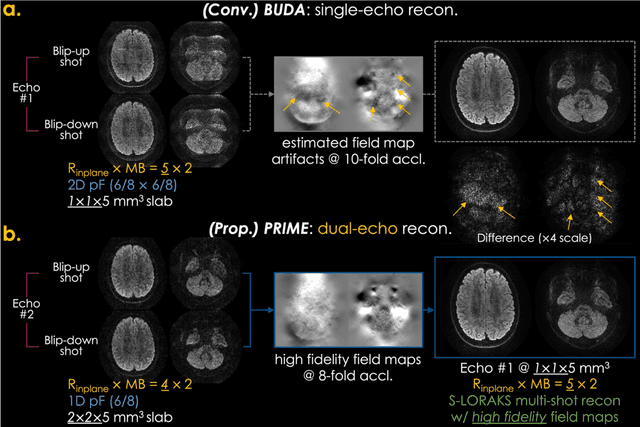
Abstract:Purpose: To develop and evaluate a new pulse sequence for highly accelerated distortion-free diffusion MRI (dMRI) by inserting an additional echo without prolonging TR, when generalized slice dithered enhanced resolution (gSlider) radiofrequency encoding is used for volumetric acquisition. Methods: A phase-reversed interleaved multi-echo acquisition (PRIME) was developed for rapid, high-resolution, and distortion-free dMRI, which includes two echoes where the first echo is for target diffusion-weighted imaging (DWI) acquisition with high-resolution and the second echo is acquired with either 1) lower-resolution for high-fidelity field map estimation, or 2) matching resolution to enable efficient diffusion relaxometry acquisitions. The sequence was evaluated on in vivo data acquired from healthy volunteers on clinical and Connectome 2.0 scanners. Results: In vivo experiments demonstrated that 1) high in-plane acceleration (Rin-plane of 5-fold with 2D partial Fourier) was achieved using the high-fidelity field maps estimated from the second echo, which was made at a lower resolution/acceleration to increase its SNR while matching the effective echo spacing of the first readout, 2) high-resolution diffusion relaxometry parameters were estimated from dual-echo PRIME data using a white matter model of multi-TE spherical mean technique (MTE-SMT), and 3) high-fidelity mesoscale DWI at 550 um isotropic resolution could be obtained in vivo by capitalizing on the high-performance gradients of the Connectome 2.0 scanner. Conclusion: The proposed PRIME sequence enabled highly accelerated, high-resolution, and distortion-free dMRI using an additional echo without prolonging scan time when gSlider encoding is utilized.
EVENet: Evidence-based Ensemble Learning for Uncertainty-aware Brain Parcellation Using Diffusion MRI
Sep 11, 2024



Abstract:In this study, we developed an Evidence-based Ensemble Neural Network, namely EVENet, for anatomical brain parcellation using diffusion MRI. The key innovation of EVENet is the design of an evidential deep learning framework to quantify predictive uncertainty at each voxel during a single inference. Using EVENet, we obtained accurate parcellation and uncertainty estimates across different datasets from healthy and clinical populations and with different imaging acquisitions. The overall network includes five parallel subnetworks, where each is dedicated to learning the FreeSurfer parcellation for a certain diffusion MRI parameter. An evidence-based ensemble methodology is then proposed to fuse the individual outputs. We perform experimental evaluations on large-scale datasets from multiple imaging sources, including high-quality diffusion MRI data from healthy adults and clinically diffusion MRI data from participants with various brain diseases (schizophrenia, bipolar disorder, attention-deficit/hyperactivity disorder, Parkinson's disease, cerebral small vessel disease, and neurosurgical patients with brain tumors). Compared to several state-of-the-art methods, our experimental results demonstrate highly improved parcellation accuracy across the multiple testing datasets despite the differences in dMRI acquisition protocols and health conditions. Furthermore, thanks to the uncertainty estimation, our EVENet approach demonstrates a good ability to detect abnormal brain regions in patients with lesions, enhancing the interpretability and reliability of the segmentation results.
A study of why we need to reassess full reference image quality assessment with medical images
May 29, 2024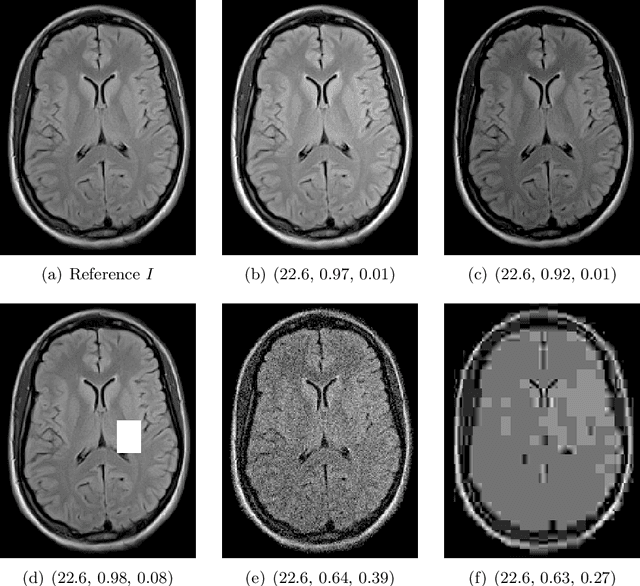
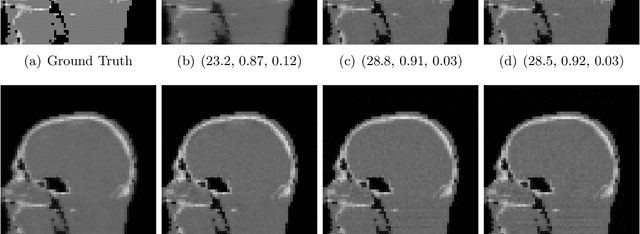
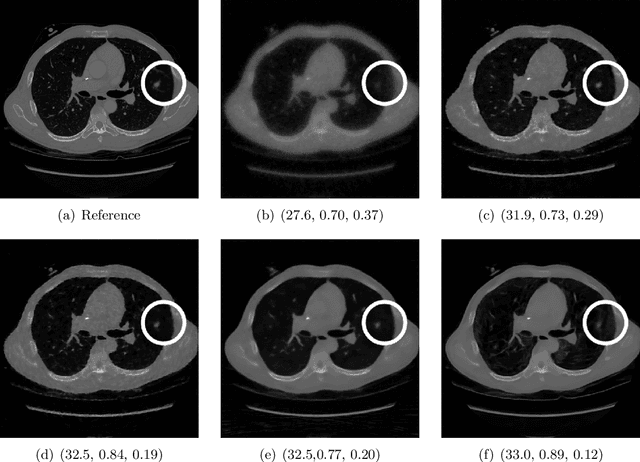
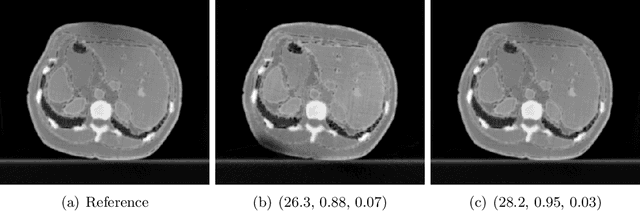
Abstract:Image quality assessment (IQA) is not just indispensable in clinical practice to ensure high standards, but also in the development stage of novel algorithms that operate on medical images with reference data. This paper provides a structured and comprehensive collection of examples where the two most common full reference (FR) image quality measures prove to be unsuitable for the assessment of novel algorithms using different kinds of medical images, including real-world MRI, CT, OCT, X-Ray, digital pathology and photoacoustic imaging data. In particular, the FR-IQA measures PSNR and SSIM are known and tested for working successfully in many natural imaging tasks, but discrepancies in medical scenarios have been noted in the literature. Inconsistencies arising in medical images are not surprising, as they have very different properties than natural images which have not been targeted nor tested in the development of the mentioned measures, and therefore might imply wrong judgement of novel methods for medical images. Therefore, improvement is urgently needed in particular in this era of AI to increase explainability, reproducibility and generalizability in machine learning for medical imaging and beyond. On top of the pitfalls we will provide ideas for future research as well as suggesting guidelines for the usage of FR-IQA measures applied to medical images.
Neural Orientation Distribution Fields for Estimation and Uncertainty Quantification in Diffusion MRI
Jul 16, 2023



Abstract:Inferring brain connectivity and structure \textit{in-vivo} requires accurate estimation of the orientation distribution function (ODF), which encodes key local tissue properties. However, estimating the ODF from diffusion MRI (dMRI) signals is a challenging inverse problem due to obstacles such as significant noise, high-dimensional parameter spaces, and sparse angular measurements. In this paper, we address these challenges by proposing a novel deep-learning based methodology for continuous estimation and uncertainty quantification of the spatially varying ODF field. We use a neural field (NF) to parameterize a random series representation of the latent ODFs, implicitly modeling the often ignored but valuable spatial correlation structures in the data, and thereby improving efficiency in sparse and noisy regimes. An analytic approximation to the posterior predictive distribution is derived which can be used to quantify the uncertainty in the ODF estimate at any spatial location, avoiding the need for expensive resampling-based approaches that are typically employed for this purpose. We present empirical evaluations on both synthetic and real in-vivo diffusion data, demonstrating the advantages of our method over existing approaches.
SlicerTMS: Interactive Real-time Visualization of Transcranial Magnetic Stimulation using Augmented Reality and Deep Learning
May 23, 2023



Abstract:Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulation approach that effectively treats various brain disorders. One of the critical factors in the success of TMS treatment is accurate coil placement, which can be challenging, especially when targeting specific brain areas for individual patients. Calculating the optimal coil placement and the resulting electric field on the brain surface can be expensive and time-consuming. We introduce SlicerTMS, a simulation method that allows the real-time visualization of the TMS electromagnetic field within the medical imaging platform 3D Slicer. Our software leverages a 3D deep neural network, supports cloud-based inference, and includes augmented reality visualization using WebXR. We evaluate the performance of SlicerTMS with multiple hardware configurations and compare it against the existing TMS visualization application SimNIBS. All our code, data, and experiments are openly available: \url{https://github.com/lorifranke/SlicerTMS}
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge