Wen Yan
Generative molecule evolution using 3D pharmacophore for efficient Structure-Based Drug Design
Jul 27, 2025



Abstract:Recent advances in generative models, particularly diffusion and auto-regressive models, have revolutionized fields like computer vision and natural language processing. However, their application to structure-based drug design (SBDD) remains limited due to critical data constraints. To address the limitation of training data for models targeting SBDD tasks, we propose an evolutionary framework named MEVO, which bridges the gap between billion-scale small molecule dataset and the scarce protein-ligand complex dataset, and effectively increase the abundance of training data for generative SBDD models. MEVO is composed of three key components: a high-fidelity VQ-VAE for molecule representation in latent space, a diffusion model for pharmacophore-guided molecule generation, and a pocket-aware evolutionary strategy for molecule optimization with physics-based scoring function. This framework efficiently generate high-affinity binders for various protein targets, validated with predicted binding affinities using free energy perturbation (FEP) methods. In addition, we showcase the capability of MEVO in designing potent inhibitors to KRAS$^{\textrm{G12D}}$, a challenging target in cancer therapeutics, with similar affinity to the known highly active inhibitor evaluated by FEP calculations. With high versatility and generalizability, MEVO offers an effective and data-efficient model for various tasks in structure-based ligand design.
Tell2Reg: Establishing spatial correspondence between images by the same language prompts
Feb 05, 2025Abstract:Spatial correspondence can be represented by pairs of segmented regions, such that the image registration networks aim to segment corresponding regions rather than predicting displacement fields or transformation parameters. In this work, we show that such a corresponding region pair can be predicted by the same language prompt on two different images using the pre-trained large multimodal models based on GroundingDINO and SAM. This enables a fully automated and training-free registration algorithm, potentially generalisable to a wide range of image registration tasks. In this paper, we present experimental results using one of the challenging tasks, registering inter-subject prostate MR images, which involves both highly variable intensity and morphology between patients. Tell2Reg is training-free, eliminating the need for costly and time-consuming data curation and labelling that was previously required for this registration task. This approach outperforms unsupervised learning-based registration methods tested, and has a performance comparable to weakly-supervised methods. Additional qualitative results are also presented to suggest that, for the first time, there is a potential correlation between language semantics and spatial correspondence, including the spatial invariance in language-prompted regions and the difference in language prompts between the obtained local and global correspondences. Code is available at https://github.com/yanwenCi/Tell2Reg.git.
SAMReg: SAM-enabled Image Registration with ROI-based Correspondence
Oct 17, 2024



Abstract:This paper describes a new spatial correspondence representation based on paired regions-of-interest (ROIs), for medical image registration. The distinct properties of the proposed ROI-based correspondence are discussed, in the context of potential benefits in clinical applications following image registration, compared with alternative correspondence-representing approaches, such as those based on sampled displacements and spatial transformation functions. These benefits include a clear connection between learning-based image registration and segmentation, which in turn motivates two cases of image registration approaches using (pre-)trained segmentation networks. Based on the segment anything model (SAM), a vision foundation model for segmentation, we develop a new registration algorithm SAMReg, which does not require any training (or training data), gradient-based fine-tuning or prompt engineering. The proposed SAMReg models are evaluated across five real-world applications, including intra-subject registration tasks with cardiac MR and lung CT, challenging inter-subject registration scenarios with prostate MR and retinal imaging, and an additional evaluation with a non-clinical example with aerial image registration. The proposed methods outperform both intensity-based iterative algorithms and DDF-predicting learning-based networks across tested metrics including Dice and target registration errors on anatomical structures, and further demonstrates competitive performance compared to weakly-supervised registration approaches that rely on fully-segmented training data. Open source code and examples are available at: https://github.com/sqhuang0103/SAMReg.git.
Data-Driven Parametrization of Molecular Mechanics Force Fields for Expansive Chemical Space Coverage
Aug 23, 2024



Abstract:A force field is a critical component in molecular dynamics simulations for computational drug discovery. It must achieve high accuracy within the constraints of molecular mechanics' (MM) limited functional forms, which offers high computational efficiency. With the rapid expansion of synthetically accessible chemical space, traditional look-up table approaches face significant challenges. In this study, we address this issue using a modern data-driven approach, developing ByteFF, an Amber-compatible force field for drug-like molecules. To create ByteFF, we generated an expansive and highly diverse molecular dataset at the B3LYP-D3(BJ)/DZVP level of theory. This dataset includes 2.4 million optimized molecular fragment geometries with analytical Hessian matrices, along with 3.2 million torsion profiles. We then trained an edge-augmented, symmetry-preserving molecular graph neural network (GNN) on this dataset, employing a carefully optimized training strategy. Our model predicts all bonded and non-bonded MM force field parameters for drug-like molecules simultaneously across a broad chemical space. ByteFF demonstrates state-of-the-art performance on various benchmark datasets, excelling in predicting relaxed geometries, torsional energy profiles, and conformational energies and forces. Its exceptional accuracy and expansive chemical space coverage make ByteFF a valuable tool for multiple stages of computational drug discovery.
One registration is worth two segmentations
May 17, 2024



Abstract:The goal of image registration is to establish spatial correspondence between two or more images, traditionally through dense displacement fields (DDFs) or parametric transformations (e.g., rigid, affine, and splines). Rethinking the existing paradigms of achieving alignment via spatial transformations, we uncover an alternative but more intuitive correspondence representation: a set of corresponding regions-of-interest (ROI) pairs, which we demonstrate to have sufficient representational capability as other correspondence representation methods.Further, it is neither necessary nor sufficient for these ROIs to hold specific anatomical or semantic significance. In turn, we formulate image registration as searching for the same set of corresponding ROIs from both moving and fixed images - in other words, two multi-class segmentation tasks on a pair of images. For a general-purpose and practical implementation, we integrate the segment anything model (SAM) into our proposed algorithms, resulting in a SAM-enabled registration (SAMReg) that does not require any training data, gradient-based fine-tuning or engineered prompts. We experimentally show that the proposed SAMReg is capable of segmenting and matching multiple ROI pairs, which establish sufficiently accurate correspondences, in three clinical applications of registering prostate MR, cardiac MR and abdominal CT images. Based on metrics including Dice and target registration errors on anatomical structures, the proposed registration outperforms both intensity-based iterative algorithms and DDF-predicting learning-based networks, even yielding competitive performance with weakly-supervised registration which requires fully-segmented training data.
BAMBOO: a predictive and transferable machine learning force field framework for liquid electrolyte development
Apr 12, 2024Abstract:Despite the widespread applications of machine learning force field (MLFF) on solids and small molecules, there is a notable gap in applying MLFF to complex liquid electrolytes. In this work, we introduce BAMBOO (ByteDance AI Molecular Simulation Booster), a novel framework for molecular dynamics (MD) simulations, with a demonstration of its capabilities in the context of liquid electrolytes for lithium batteries. We design a physics-inspired graph equivariant transformer architecture as the backbone of BAMBOO to learn from quantum mechanical simulations. Additionally, we pioneer an ensemble knowledge distillation approach and apply it on MLFFs to improve the stability of MD simulations. Finally, we propose the density alignment algorithm to align BAMBOO with experimental measurements. BAMBOO demonstrates state-of-the-art accuracy in predicting key electrolyte properties such as density, viscosity, and ionic conductivity across various solvents and salt combinations. Our current model, trained on more than 15 chemical species, achieves the average density error of 0.01 g/cm$^3$ on various compositions compared with experimental data. Moreover, our model demonstrates transferability to molecules not included in the quantum mechanical dataset. We envision this work as paving the way to a "universal MLFF" capable of simulating properties of common organic liquids.
Weakly supervised localisation of prostate cancer using reinforcement learning for bi-parametric MR images
Feb 21, 2024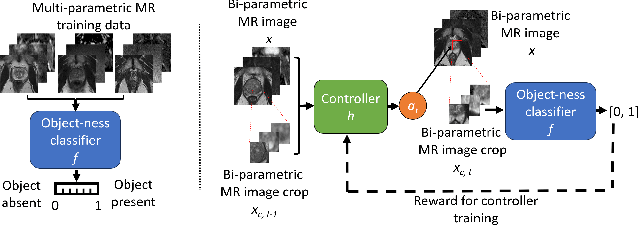

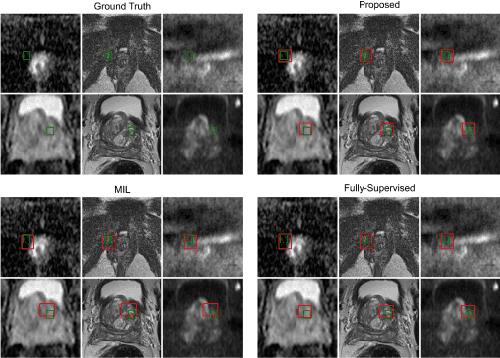
Abstract:In this paper we propose a reinforcement learning based weakly supervised system for localisation. We train a controller function to localise regions of interest within an image by introducing a novel reward definition that utilises non-binarised classification probability, generated by a pre-trained binary classifier which classifies object presence in images or image crops. The object-presence classifier may then inform the controller of its localisation quality by quantifying the likelihood of the image containing an object. Such an approach allows us to minimize any potential labelling or human bias propagated via human labelling for fully supervised localisation. We evaluate our proposed approach for a task of cancerous lesion localisation on a large dataset of real clinical bi-parametric MR images of the prostate. Comparisons to the commonly used multiple-instance learning weakly supervised localisation and to a fully supervised baseline show that our proposed method outperforms the multi-instance learning and performs comparably to fully-supervised learning, using only image-level classification labels for training.
Semi-weakly-supervised neural network training for medical image registration
Feb 16, 2024



Abstract:For training registration networks, weak supervision from segmented corresponding regions-of-interest (ROIs) have been proven effective for (a) supplementing unsupervised methods, and (b) being used independently in registration tasks in which unsupervised losses are unavailable or ineffective. This correspondence-informing supervision entails cost in annotation that requires significant specialised effort. This paper describes a semi-weakly-supervised registration pipeline that improves the model performance, when only a small corresponding-ROI-labelled dataset is available, by exploiting unlabelled image pairs. We examine two types of augmentation methods by perturbation on network weights and image resampling, such that consistency-based unsupervised losses can be applied on unlabelled data. The novel WarpDDF and RegCut approaches are proposed to allow commutative perturbation between an image pair and the predicted spatial transformation (i.e. respective input and output of registration networks), distinct from existing perturbation methods for classification or segmentation. Experiments using 589 male pelvic MR images, labelled with eight anatomical ROIs, show the improvement in registration performance and the ablated contributions from the individual strategies. Furthermore, this study attempts to construct one of the first computational atlases for pelvic structures, enabled by registering inter-subject MRs, and quantifies the significant differences due to the proposed semi-weak supervision with a discussion on the potential clinical use of example atlas-derived statistics.
Combiner and HyperCombiner Networks: Rules to Combine Multimodality MR Images for Prostate Cancer Localisation
Jul 17, 2023



Abstract:One of the distinct characteristics in radiologists' reading of multiparametric prostate MR scans, using reporting systems such as PI-RADS v2.1, is to score individual types of MR modalities, T2-weighted, diffusion-weighted, and dynamic contrast-enhanced, and then combine these image-modality-specific scores using standardised decision rules to predict the likelihood of clinically significant cancer. This work aims to demonstrate that it is feasible for low-dimensional parametric models to model such decision rules in the proposed Combiner networks, without compromising the accuracy of predicting radiologic labels: First, it is shown that either a linear mixture model or a nonlinear stacking model is sufficient to model PI-RADS decision rules for localising prostate cancer. Second, parameters of these (generalised) linear models are proposed as hyperparameters, to weigh multiple networks that independently represent individual image modalities in the Combiner network training, as opposed to end-to-end modality ensemble. A HyperCombiner network is developed to train a single image segmentation network that can be conditioned on these hyperparameters during inference, for much improved efficiency. Experimental results based on data from 850 patients, for the application of automating radiologist labelling multi-parametric MR, compare the proposed combiner networks with other commonly-adopted end-to-end networks. Using the added advantages of obtaining and interpreting the modality combining rules, in terms of the linear weights or odds-ratios on individual image modalities, three clinical applications are presented for prostate cancer segmentation, including modality availability assessment, importance quantification and rule discovery.
Machine Learning Force Fields with Data Cost Aware Training
Jun 05, 2023



Abstract:Machine learning force fields (MLFF) have been proposed to accelerate molecular dynamics (MD) simulation, which finds widespread applications in chemistry and biomedical research. Even for the most data-efficient MLFFs, reaching chemical accuracy can require hundreds of frames of force and energy labels generated by expensive quantum mechanical algorithms, which may scale as $O(n^3)$ to $O(n^7)$, with $n$ proportional to the number of basis functions. To address this issue, we propose a multi-stage computational framework -- ASTEROID, which lowers the data cost of MLFFs by leveraging a combination of cheap inaccurate data and expensive accurate data. The motivation behind ASTEROID is that inaccurate data, though incurring large bias, can help capture the sophisticated structures of the underlying force field. Therefore, we first train a MLFF model on a large amount of inaccurate training data, employing a bias-aware loss function to prevent the model from overfitting tahe potential bias of this data. We then fine-tune the obtained model using a small amount of accurate training data, which preserves the knowledge learned from the inaccurate training data while significantly improving the model's accuracy. Moreover, we propose a variant of ASTEROID based on score matching for the setting where the inaccurate training data are unlabeled. Extensive experiments on MD datasets and downstream tasks validate the efficacy of ASTEROID. Our code and data are available at https://github.com/abukharin3/asteroid.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge