Junyan Wu
Kimi K2.5: Visual Agentic Intelligence
Feb 02, 2026Abstract:We introduce Kimi K2.5, an open-source multimodal agentic model designed to advance general agentic intelligence. K2.5 emphasizes the joint optimization of text and vision so that two modalities enhance each other. This includes a series of techniques such as joint text-vision pre-training, zero-vision SFT, and joint text-vision reinforcement learning. Building on this multimodal foundation, K2.5 introduces Agent Swarm, a self-directed parallel agent orchestration framework that dynamically decomposes complex tasks into heterogeneous sub-problems and executes them concurrently. Extensive evaluations show that Kimi K2.5 achieves state-of-the-art results across various domains including coding, vision, reasoning, and agentic tasks. Agent Swarm also reduces latency by up to $4.5\times$ over single-agent baselines. We release the post-trained Kimi K2.5 model checkpoint to facilitate future research and real-world applications of agentic intelligence.
A Multimodal Deviation Perceiving Framework for Weakly-Supervised Temporal Forgery Localization
Jul 22, 2025Abstract:Current researches on Deepfake forensics often treat detection as a classification task or temporal forgery localization problem, which are usually restrictive, time-consuming, and challenging to scale for large datasets. To resolve these issues, we present a multimodal deviation perceiving framework for weakly-supervised temporal forgery localization (MDP), which aims to identify temporal partial forged segments using only video-level annotations. The MDP proposes a novel multimodal interaction mechanism (MI) and an extensible deviation perceiving loss to perceive multimodal deviation, which achieves the refined start and end timestamps localization of forged segments. Specifically, MI introduces a temporal property preserving cross-modal attention to measure the relevance between the visual and audio modalities in the probabilistic embedding space. It could identify the inter-modality deviation and construct comprehensive video features for temporal forgery localization. To explore further temporal deviation for weakly-supervised learning, an extensible deviation perceiving loss has been proposed, aiming at enlarging the deviation of adjacent segments of the forged samples and reducing that of genuine samples. Extensive experiments demonstrate the effectiveness of the proposed framework and achieve comparable results to fully-supervised approaches in several evaluation metrics.
VoiceCloak: A Multi-Dimensional Defense Framework against Unauthorized Diffusion-based Voice Cloning
May 18, 2025Abstract:Diffusion Models (DMs) have achieved remarkable success in realistic voice cloning (VC), while they also increase the risk of malicious misuse. Existing proactive defenses designed for traditional VC models aim to disrupt the forgery process, but they have been proven incompatible with DMs due to the intricate generative mechanisms of diffusion. To bridge this gap, we introduce VoiceCloak, a multi-dimensional proactive defense framework with the goal of obfuscating speaker identity and degrading perceptual quality in potential unauthorized VC. To achieve these goals, we conduct a focused analysis to identify specific vulnerabilities within DMs, allowing VoiceCloak to disrupt the cloning process by introducing adversarial perturbations into the reference audio. Specifically, to obfuscate speaker identity, VoiceCloak first targets speaker identity by distorting representation learning embeddings to maximize identity variation, which is guided by auditory perception principles. Additionally, VoiceCloak disrupts crucial conditional guidance processes, particularly attention context, thereby preventing the alignment of vocal characteristics that are essential for achieving convincing cloning. Then, to address the second objective, VoiceCloak introduces score magnitude amplification to actively steer the reverse trajectory away from the generation of high-quality speech. Noise-guided semantic corruption is further employed to disrupt structural speech semantics captured by DMs, degrading output quality. Extensive experiments highlight VoiceCloak's outstanding defense success rate against unauthorized diffusion-based voice cloning. Audio samples of VoiceCloak are available at https://voice-cloak.github.io/VoiceCloak/.
Weakly-supervised Audio Temporal Forgery Localization via Progressive Audio-language Co-learning Network
May 03, 2025Abstract:Audio temporal forgery localization (ATFL) aims to find the precise forgery regions of the partial spoof audio that is purposefully modified. Existing ATFL methods rely on training efficient networks using fine-grained annotations, which are obtained costly and challenging in real-world scenarios. To meet this challenge, in this paper, we propose a progressive audio-language co-learning network (LOCO) that adopts co-learning and self-supervision manners to prompt localization performance under weak supervision scenarios. Specifically, an audio-language co-learning module is first designed to capture forgery consensus features by aligning semantics from temporal and global perspectives. In this module, forgery-aware prompts are constructed by using utterance-level annotations together with learnable prompts, which can incorporate semantic priors into temporal content features dynamically. In addition, a forgery localization module is applied to produce forgery proposals based on fused forgery-class activation sequences. Finally, a progressive refinement strategy is introduced to generate pseudo frame-level labels and leverage supervised semantic contrastive learning to amplify the semantic distinction between real and fake content, thereby continuously optimizing forgery-aware features. Extensive experiments show that the proposed LOCO achieves SOTA performance on three public benchmarks.
Coarse-to-Fine Proposal Refinement Framework for Audio Temporal Forgery Detection and Localization
Jul 23, 2024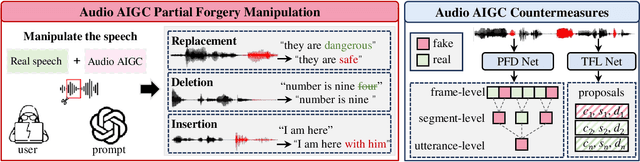
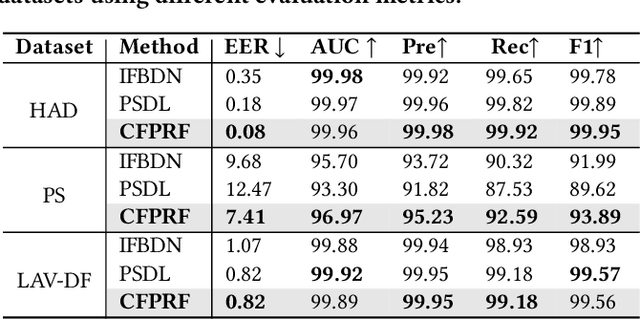
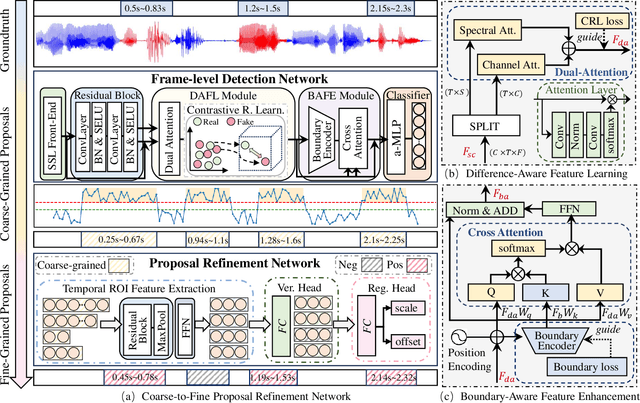
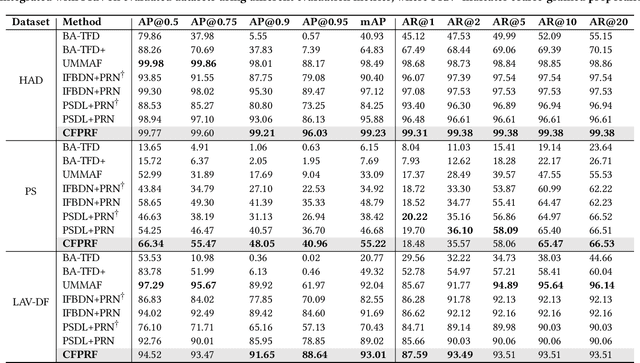
Abstract:Recently, a novel form of audio partial forgery has posed challenges to its forensics, requiring advanced countermeasures to detect subtle forgery manipulations within long-duration audio. However, existing countermeasures still serve a classification purpose and fail to perform meaningful analysis of the start and end timestamps of partial forgery segments. To address this challenge, we introduce a novel coarse-to-fine proposal refinement framework (CFPRF) that incorporates a frame-level detection network (FDN) and a proposal refinement network (PRN) for audio temporal forgery detection and localization. Specifically, the FDN aims to mine informative inconsistency cues between real and fake frames to obtain discriminative features that are beneficial for roughly indicating forgery regions. The PRN is responsible for predicting confidence scores and regression offsets to refine the coarse-grained proposals derived from the FDN. To learn robust discriminative features, we devise a difference-aware feature learning (DAFL) module guided by contrastive representation learning to enlarge the sensitive differences between different frames induced by minor manipulations. We further design a boundary-aware feature enhancement (BAFE) module to capture the contextual information of multiple transition boundaries and guide the interaction between boundary information and temporal features via a cross-attention mechanism. Extensive experiments show that our CFPRF achieves state-of-the-art performance on various datasets, including LAV-DF, ASVS2019PS, and HAD.
Deep learning model trained on mobile phone-acquired frozen section images effectively detects basal cell carcinoma
Nov 22, 2020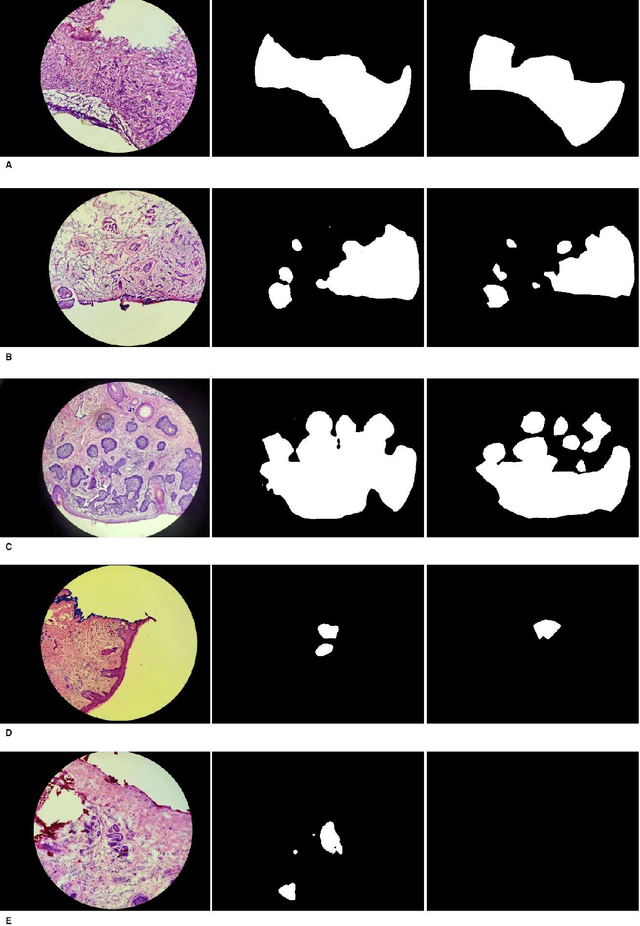
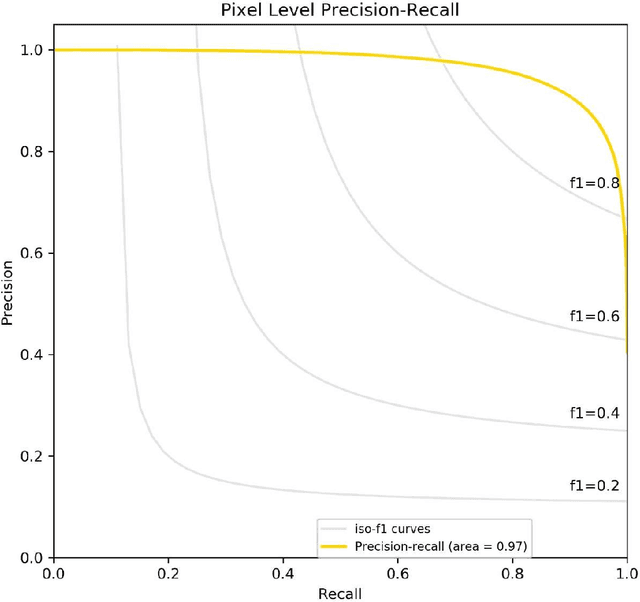
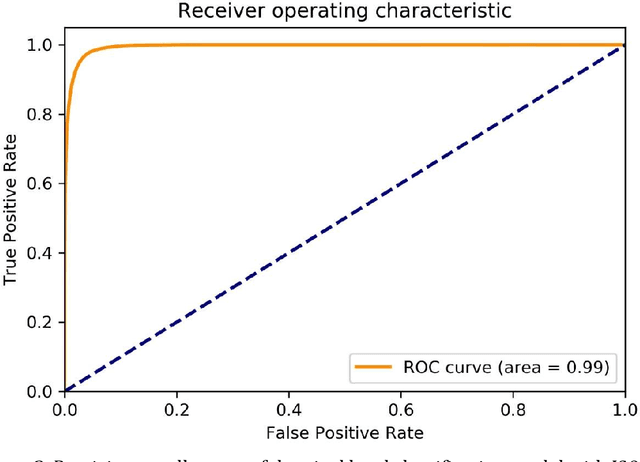
Abstract:Background: Margin assessment of basal cell carcinoma using the frozen section is a common task of pathology intraoperative consultation. Although frequently straight-forward, the determination of the presence or absence of basal cell carcinoma on the tissue sections can sometimes be challenging. We explore if a deep learning model trained on mobile phone-acquired frozen section images can have adequate performance for future deployment. Materials and Methods: One thousand two hundred and forty-one (1241) images of frozen sections performed for basal cell carcinoma margin status were acquired using mobile phones. The photos were taken at 100x magnification (10x objective). The images were downscaled from a 4032 x 3024 pixel resolution to 576 x 432 pixel resolution. Semantic segmentation algorithm Deeplab V3 with Xception backbone was used for model training. Results: The model uses an image as input and produces a 2-dimensional black and white output of prediction of the same dimension; the areas determined to be basal cell carcinoma were displayed with white color, in a black background. Any output with the number of white pixels exceeding 0.5% of the total number of pixels is deemed positive for basal cell carcinoma. On the test set, the model achieves area under curve of 0.99 for receiver operator curve and 0.97 for precision-recall curve at the pixel level. The accuracy of classification at the slide level is 96%. Conclusions: The deep learning model trained with mobile phone images shows satisfactory performance characteristics, and thus demonstrates the potential for deploying as a mobile phone app to assist in frozen section interpretation in real time.
Learning Differential Diagnosis of Skin Conditions with Co-occurrence Supervision using Graph Convolutional Networks
Jul 13, 2020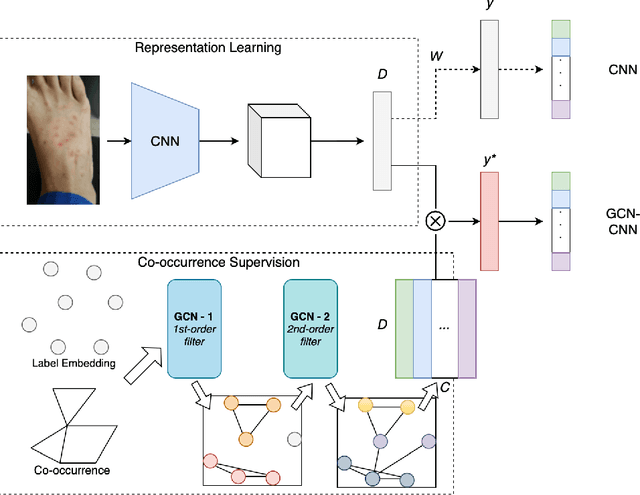
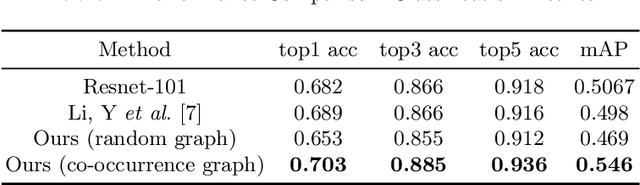


Abstract:Skin conditions are reported the 4th leading cause of nonfatal disease burden worldwide. However, given the colossal spectrum of skin disorders defined clinically and shortage in dermatology expertise, diagnosing skin conditions in a timely and accurate manner remains a challenging task. Using computer vision technologies, a deep learning system has proven effective assisting clinicians in image diagnostics of radiology, ophthalmology and more. In this paper, we propose a deep learning system (DLS) that may predict differential diagnosis of skin conditions using clinical images. Our DLS formulates the differential diagnostics as a multi-label classification task over 80 conditions when only incomplete image labels are available. We tackle the label incompleteness problem by combining a classification network with a Graph Convolutional Network (GCN) that characterizes label co-occurrence and effectively regularizes it towards a sparse representation. Our approach is demonstrated on 136,462 clinical images and concludes that the classification accuracy greatly benefit from the Co-occurrence supervision. Our DLS achieves 93.6% top-5 accuracy on 12,378 test images and consistently outperform the baseline classification network.
REFUGE Challenge: A Unified Framework for Evaluating Automated Methods for Glaucoma Assessment from Fundus Photographs
Oct 08, 2019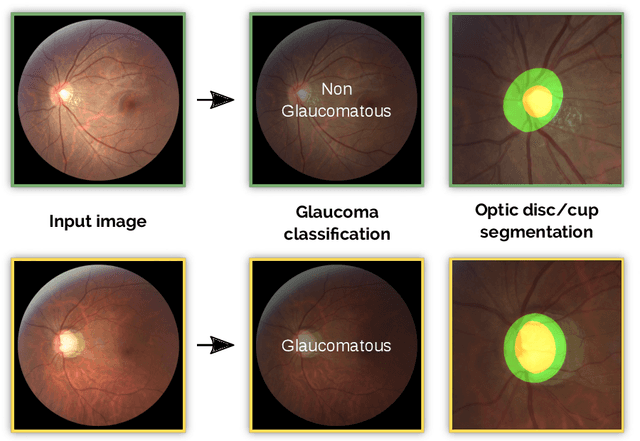
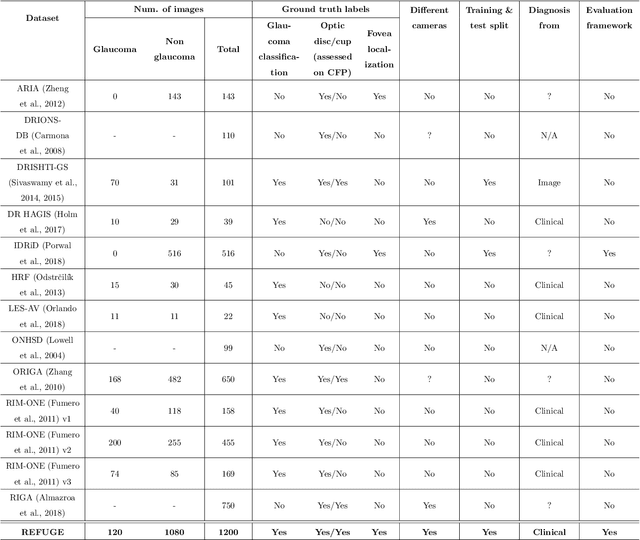
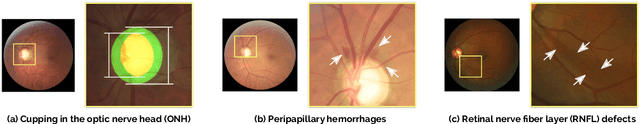
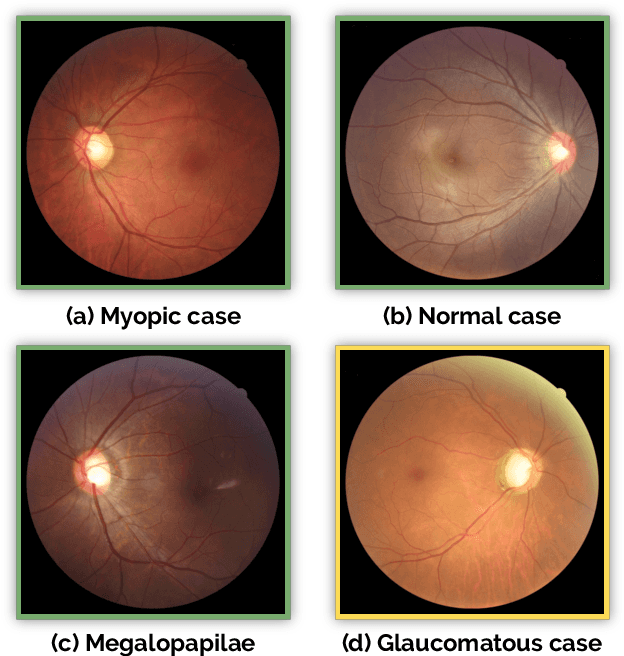
Abstract:Glaucoma is one of the leading causes of irreversible but preventable blindness in working age populations. Color fundus photography (CFP) is the most cost-effective imaging modality to screen for retinal disorders. However, its application to glaucoma has been limited to the computation of a few related biomarkers such as the vertical cup-to-disc ratio. Deep learning approaches, although widely applied for medical image analysis, have not been extensively used for glaucoma assessment due to the limited size of the available data sets. Furthermore, the lack of a standardize benchmark strategy makes difficult to compare existing methods in a uniform way. In order to overcome these issues we set up the Retinal Fundus Glaucoma Challenge, REFUGE (\url{https://refuge.grand-challenge.org}), held in conjunction with MICCAI 2018. The challenge consisted of two primary tasks, namely optic disc/cup segmentation and glaucoma classification. As part of REFUGE, we have publicly released a data set of 1200 fundus images with ground truth segmentations and clinical glaucoma labels, currently the largest existing one. We have also built an evaluation framework to ease and ensure fairness in the comparison of different models, encouraging the development of novel techniques in the field. 12 teams qualified and participated in the online challenge. This paper summarizes their methods and analyzes their corresponding results. In particular, we observed that two of the top-ranked teams outperformed two human experts in the glaucoma classification task. Furthermore, the segmentation results were in general consistent with the ground truth annotations, with complementary outcomes that can be further exploited by ensembling the results.
What evidence does deep learning model use to classify Skin Lesions?
Nov 02, 2018
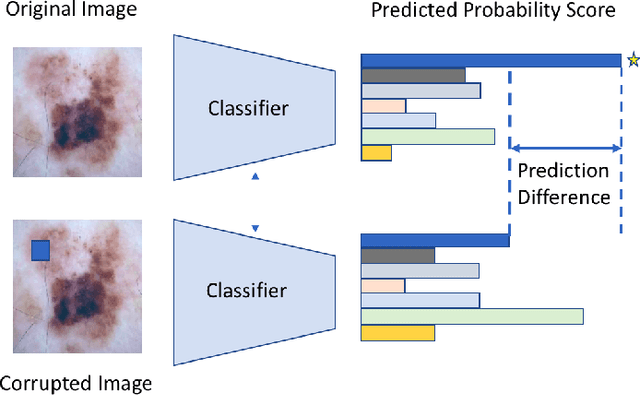
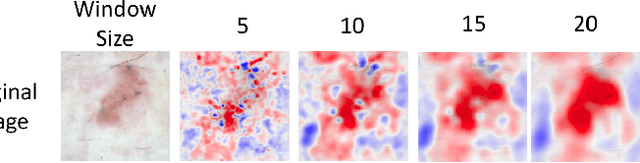
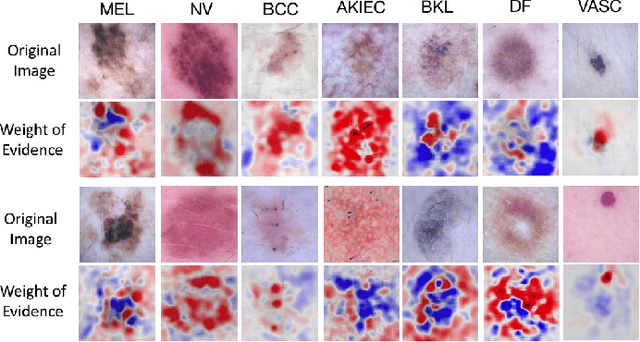
Abstract:Melanoma is a type of skin cancer with the most rapidly increasing incidence. Early detection of melanoma using dermoscopy images significantly increases patients' survival rate. However, accurately classifying skin lesions, especially in the early stage, is extremely challenging via dermatologists' observation. Hence, the discovery of reliable biomarkers for melanoma diagnosis will be meaningful. Recent years, deep learning empowered computer-assisted diagnosis has been shown its value in medical imaging-based decision making. However, lots of research focus on improving disease detection accuracy but not exploring the evidence of pathology. In this paper, we propose a method to interpret the deep learning classification findings. Firstly, we propose an accurate neural network architecture to classify skin lesion. Secondly, we utilize a prediction difference analysis method that examining each patch on the image through patch wised corrupting for detecting the biomarkers. Lastly, we validate that our biomarker findings are corresponding to the patterns in the literature. The findings might be significant to guide clinical diagnosis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge