Karel van Keer
REFUGE Challenge: A Unified Framework for Evaluating Automated Methods for Glaucoma Assessment from Fundus Photographs
Oct 08, 2019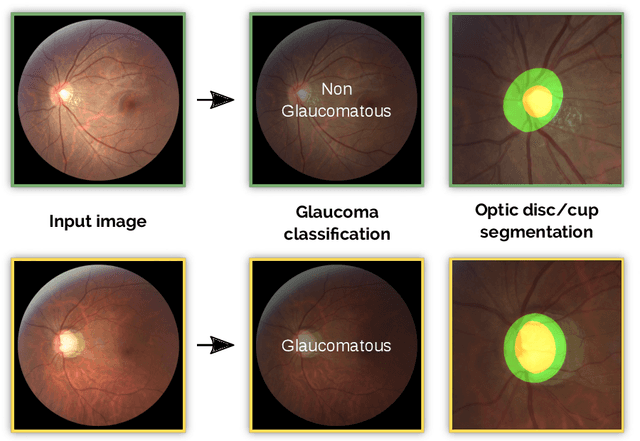
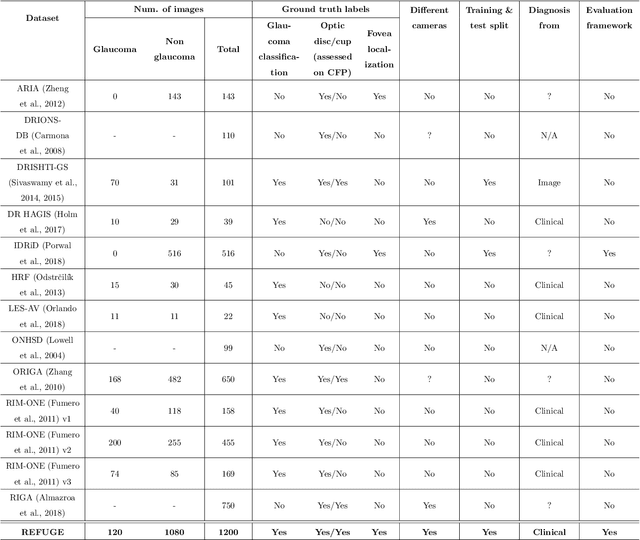
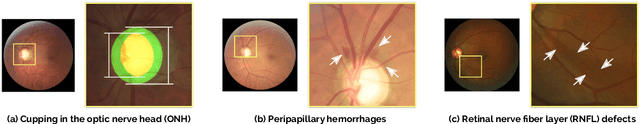
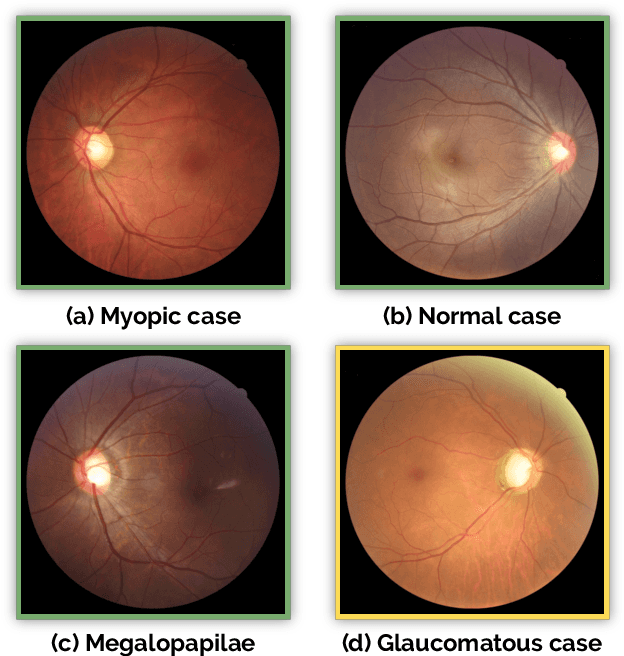
Abstract:Glaucoma is one of the leading causes of irreversible but preventable blindness in working age populations. Color fundus photography (CFP) is the most cost-effective imaging modality to screen for retinal disorders. However, its application to glaucoma has been limited to the computation of a few related biomarkers such as the vertical cup-to-disc ratio. Deep learning approaches, although widely applied for medical image analysis, have not been extensively used for glaucoma assessment due to the limited size of the available data sets. Furthermore, the lack of a standardize benchmark strategy makes difficult to compare existing methods in a uniform way. In order to overcome these issues we set up the Retinal Fundus Glaucoma Challenge, REFUGE (\url{https://refuge.grand-challenge.org}), held in conjunction with MICCAI 2018. The challenge consisted of two primary tasks, namely optic disc/cup segmentation and glaucoma classification. As part of REFUGE, we have publicly released a data set of 1200 fundus images with ground truth segmentations and clinical glaucoma labels, currently the largest existing one. We have also built an evaluation framework to ease and ensure fairness in the comparison of different models, encouraging the development of novel techniques in the field. 12 teams qualified and participated in the online challenge. This paper summarizes their methods and analyzes their corresponding results. In particular, we observed that two of the top-ranked teams outperformed two human experts in the glaucoma classification task. Furthermore, the segmentation results were in general consistent with the ground truth annotations, with complementary outcomes that can be further exploited by ensembling the results.
Towards a glaucoma risk index based on simulated hemodynamics from fundus images
Oct 25, 2018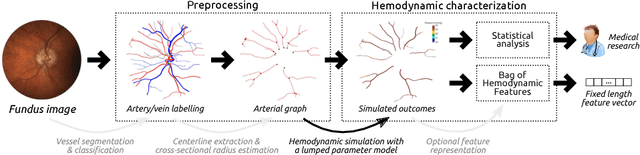
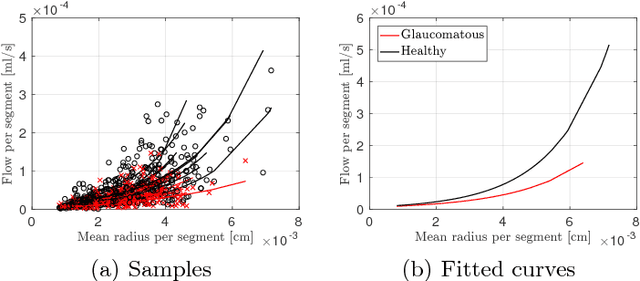
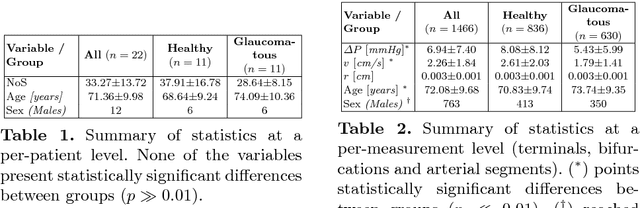
Abstract:Glaucoma is the leading cause of irreversible but preventable blindness in the world. Its major treatable risk factor is the intra-ocular pressure, although other biomarkers are being explored to improve the understanding of the pathophysiology of the disease. It has been recently observed that glaucoma induces changes in the ocular hemodynamics. However, its effects on the functional behavior of the retinal arterioles have not been studied yet. In this paper we propose a first approach for characterizing those changes using computational hemodynamics. The retinal blood flow is simulated using a 0D model for a steady, incompressible non Newtonian fluid in rigid domains. The simulation is performed on patient-specific arterial trees extracted from fundus images. We also propose a novel feature representation technique to comprise the outcomes of the simulation stage into a fixed length feature vector that can be used for classification studies. Our experiments on a new database of fundus images show that our approach is able to capture representative changes in the hemodynamics of glaucomatous patients. Code and data are publicly available in https://ignaciorlando.github.io.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge