M. S.
Math
Large Language Model-Powered Conversational Agent Delivering Problem-Solving Therapy (PST) for Family Caregivers: Enhancing Empathy and Therapeutic Alliance Using In-Context Learning
Jun 13, 2025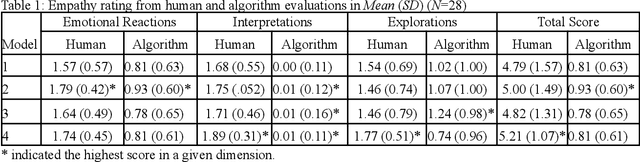
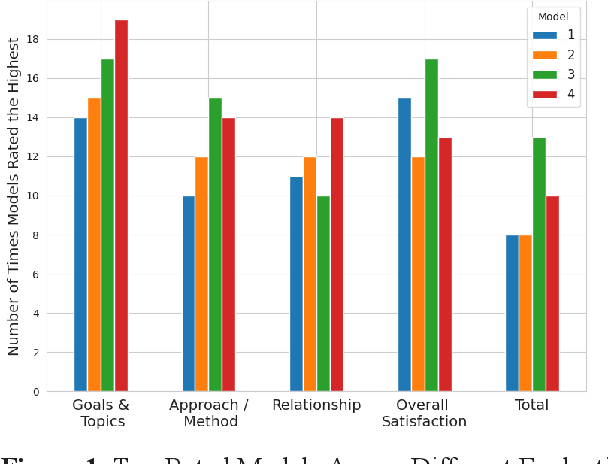
Abstract:Family caregivers often face substantial mental health challenges due to their multifaceted roles and limited resources. This study explored the potential of a large language model (LLM)-powered conversational agent to deliver evidence-based mental health support for caregivers, specifically Problem-Solving Therapy (PST) integrated with Motivational Interviewing (MI) and Behavioral Chain Analysis (BCA). A within-subject experiment was conducted with 28 caregivers interacting with four LLM configurations to evaluate empathy and therapeutic alliance. The best-performing models incorporated Few-Shot and Retrieval-Augmented Generation (RAG) prompting techniques, alongside clinician-curated examples. The models showed improved contextual understanding and personalized support, as reflected by qualitative responses and quantitative ratings on perceived empathy and therapeutic alliances. Participants valued the model's ability to validate emotions, explore unexpressed feelings, and provide actionable strategies. However, balancing thorough assessment with efficient advice delivery remains a challenge. This work highlights the potential of LLMs in delivering empathetic and tailored support for family caregivers.
Deep learning model trained on mobile phone-acquired frozen section images effectively detects basal cell carcinoma
Nov 22, 2020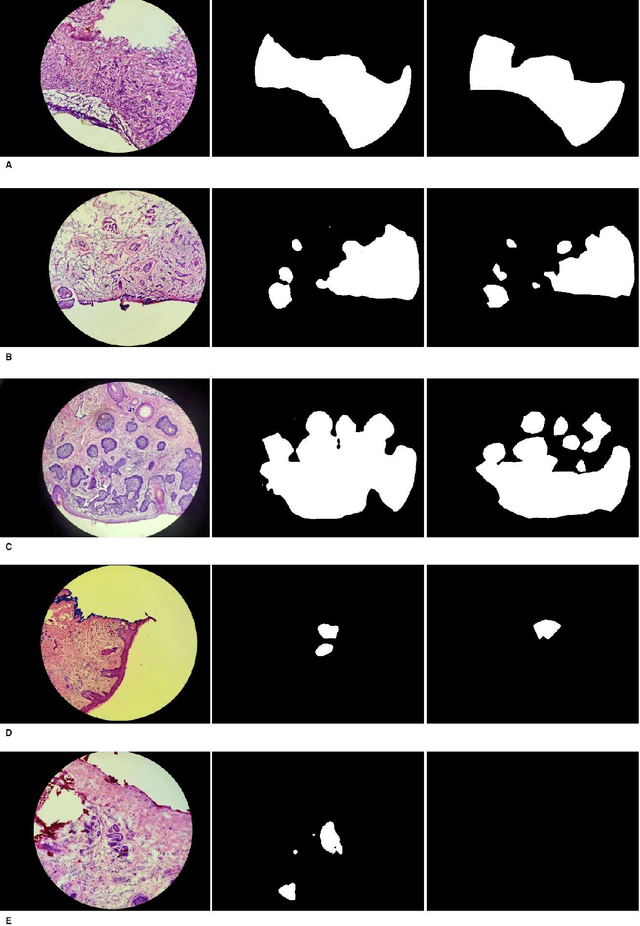
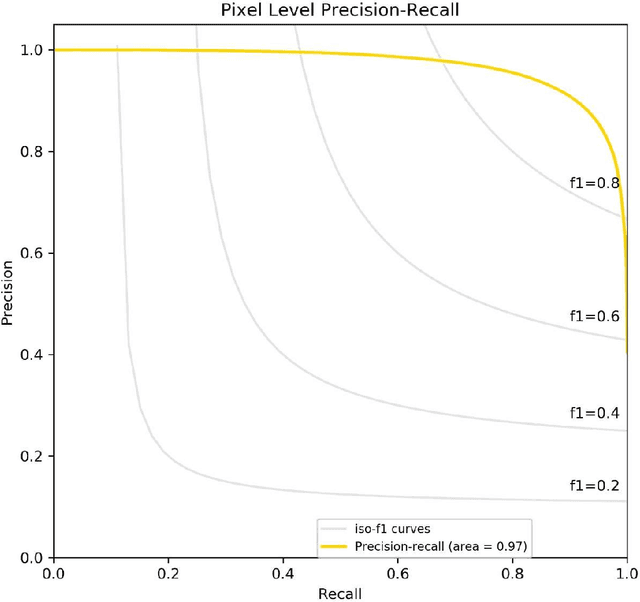
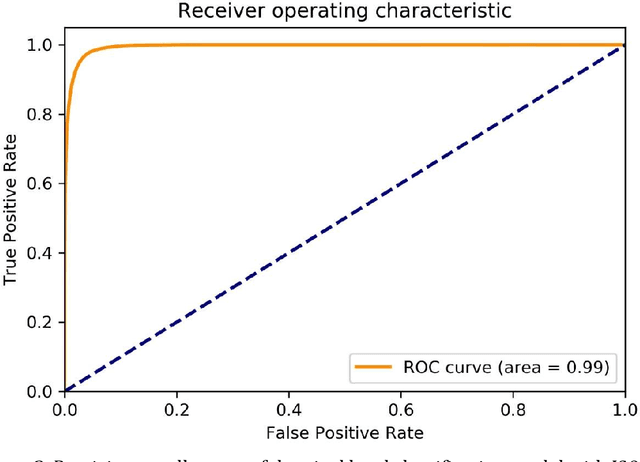
Abstract:Background: Margin assessment of basal cell carcinoma using the frozen section is a common task of pathology intraoperative consultation. Although frequently straight-forward, the determination of the presence or absence of basal cell carcinoma on the tissue sections can sometimes be challenging. We explore if a deep learning model trained on mobile phone-acquired frozen section images can have adequate performance for future deployment. Materials and Methods: One thousand two hundred and forty-one (1241) images of frozen sections performed for basal cell carcinoma margin status were acquired using mobile phones. The photos were taken at 100x magnification (10x objective). The images were downscaled from a 4032 x 3024 pixel resolution to 576 x 432 pixel resolution. Semantic segmentation algorithm Deeplab V3 with Xception backbone was used for model training. Results: The model uses an image as input and produces a 2-dimensional black and white output of prediction of the same dimension; the areas determined to be basal cell carcinoma were displayed with white color, in a black background. Any output with the number of white pixels exceeding 0.5% of the total number of pixels is deemed positive for basal cell carcinoma. On the test set, the model achieves area under curve of 0.99 for receiver operator curve and 0.97 for precision-recall curve at the pixel level. The accuracy of classification at the slide level is 96%. Conclusions: The deep learning model trained with mobile phone images shows satisfactory performance characteristics, and thus demonstrates the potential for deploying as a mobile phone app to assist in frozen section interpretation in real time.
Intracranial Hemorrhage Segmentation Using Deep Convolutional Model
Nov 15, 2019
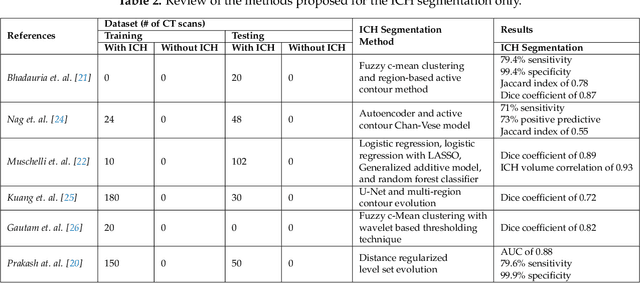
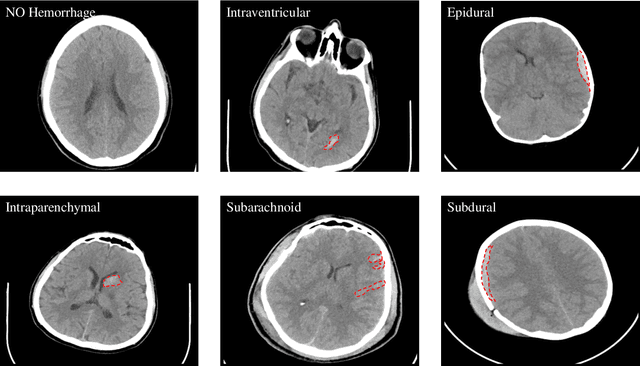
Abstract:Traumatic brain injuries could cause intracranial hemorrhage (ICH). ICH could lead to disability or death if it is not accurately diagnosed and treated in a time-sensitive procedure. The current clinical protocol to diagnose ICH is examining Computerized Tomography (CT) scans by radiologists to detect ICH and localize its regions. However, this process relies heavily on the availability of an experienced radiologist. In this paper, we designed a study protocol to collect a dataset of 82 CT scans of subjects with traumatic brain injury. Later, the ICH regions were manually delineated in each slice by a consensus decision of two radiologists. Recently, fully convolutional networks (FCN) have shown to be successful in medical image segmentation. We developed a deep FCN, called U-Net, to segment the ICH regions from the CT scans in a fully automated manner. The method achieved a Dice coefficient of 0.31 for the ICH segmentation based on 5-fold cross-validation. The dataset is publicly available online at PhysioNet repository for future analysis and comparison.
Generative Adversarial Networks Synthesize Realistic OCT Images of the Retina
Feb 18, 2019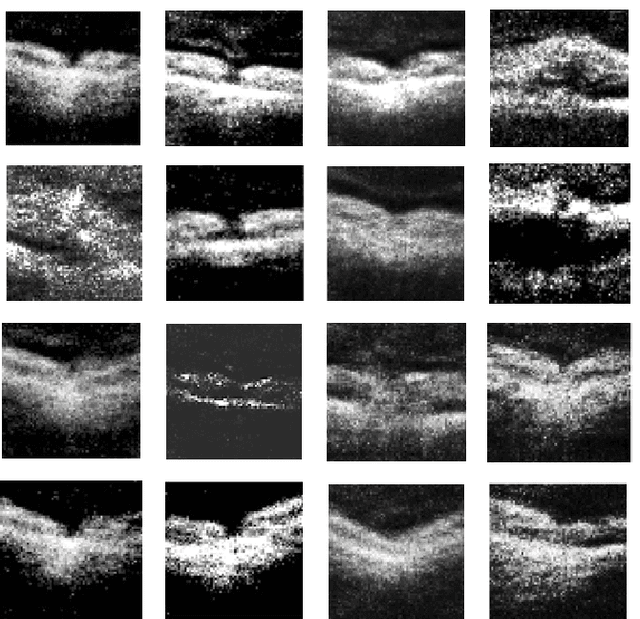
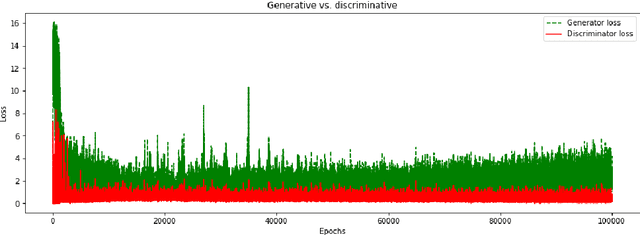
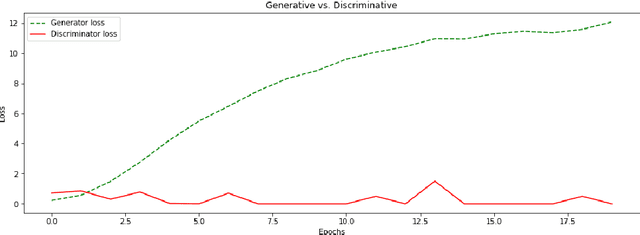
Abstract:We report, to our knowledge, the first end-to-end application of Generative Adversarial Networks (GANs) towards the synthesis of Optical Coherence Tomography (OCT) images of the retina. Generative models have gained recent attention for the increasingly realistic images they can synthesize, given a sampling of a data type. In this paper, we apply GANs to a sampling distribution of OCTs of the retina. We observe the synthesis of realistic OCT images depicting recognizable pathology such as macular holes, choroidal neovascular membranes, myopic degeneration, cystoid macular edema, and central serous retinopathy amongst others. This represents the first such report of its kind. Potential applications of this new technology include for surgical simulation, for treatment planning, for disease prognostication, and for accelerating the development of new drugs and surgical procedures to treat retinal disease.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge