Roxane Licandro
Preserving Marker Specificity with Lightweight Channel-Independent Representation Learning
Dec 17, 2025Abstract:Multiplexed tissue imaging measures dozens of protein markers per cell, yet most deep learning models still apply early channel fusion, assuming shared structure across markers. We investigate whether preserving marker independence, combined with deliberately shallow architectures, provides a more suitable inductive bias for self-supervised representation learning in multiplex data than increasing model scale. Using a Hodgkin lymphoma CODEX dataset with 145,000 cells and 49 markers, we compare standard early-fusion CNNs with channel-separated architectures, including a marker-aware baseline and our novel shallow Channel-Independent Model (CIM-S) with 5.5K parameters. After contrastive pretraining and linear evaluation, early-fusion models show limited ability to retain marker-specific information and struggle particularly with rare-cell discrimination. Channel-independent architectures, and CIM-S in particular, achieve substantially stronger representations despite their compact size. These findings are consistent across multiple self-supervised frameworks, remain stable across augmentation settings, and are reproducible across both the 49-marker and reduced 18-marker settings. These results show that lightweight, channel-independent architectures can match or surpass deep early-fusion CNNs and foundation models for multiplex representation learning. Code is available at https://github.com/SimonBon/CIM-S.
Conditional Fetal Brain Atlas Learning for Automatic Tissue Segmentation
Aug 06, 2025Abstract:Magnetic Resonance Imaging (MRI) of the fetal brain has become a key tool for studying brain development in vivo. Yet, its assessment remains challenging due to variability in brain maturation, imaging protocols, and uncertain estimates of Gestational Age (GA). To overcome these, brain atlases provide a standardized reference framework that facilitates objective evaluation and comparison across subjects by aligning the atlas and subjects in a common coordinate system. In this work, we introduce a novel deep-learning framework for generating continuous, age-specific fetal brain atlases for real-time fetal brain tissue segmentation. The framework combines a direct registration model with a conditional discriminator. Trained on a curated dataset of 219 neurotypical fetal MRIs spanning from 21 to 37 weeks of gestation. The method achieves high registration accuracy, captures dynamic anatomical changes with sharp structural detail, and robust segmentation performance with an average Dice Similarity Coefficient (DSC) of 86.3% across six brain tissues. Furthermore, volumetric analysis of the generated atlases reveals detailed neurotypical growth trajectories, providing valuable insights into the maturation of the fetal brain. This approach enables individualized developmental assessment with minimal pre-processing and real-time performance, supporting both research and clinical applications. The model code is available at https://github.com/cirmuw/fetal-brain-atlas
Advances in Automated Fetal Brain MRI Segmentation and Biometry: Insights from the FeTA 2024 Challenge
May 05, 2025



Abstract:Accurate fetal brain tissue segmentation and biometric analysis are essential for studying brain development in utero. The FeTA Challenge 2024 advanced automated fetal brain MRI analysis by introducing biometry prediction as a new task alongside tissue segmentation. For the first time, our diverse multi-centric test set included data from a new low-field (0.55T) MRI dataset. Evaluation metrics were also expanded to include the topology-specific Euler characteristic difference (ED). Sixteen teams submitted segmentation methods, most of which performed consistently across both high- and low-field scans. However, longitudinal trends indicate that segmentation accuracy may be reaching a plateau, with results now approaching inter-rater variability. The ED metric uncovered topological differences that were missed by conventional metrics, while the low-field dataset achieved the highest segmentation scores, highlighting the potential of affordable imaging systems when paired with high-quality reconstruction. Seven teams participated in the biometry task, but most methods failed to outperform a simple baseline that predicted measurements based solely on gestational age, underscoring the challenge of extracting reliable biometric estimates from image data alone. Domain shift analysis identified image quality as the most significant factor affecting model generalization, with super-resolution pipelines also playing a substantial role. Other factors, such as gestational age, pathology, and acquisition site, had smaller, though still measurable, effects. Overall, FeTA 2024 offers a comprehensive benchmark for multi-class segmentation and biometry estimation in fetal brain MRI, underscoring the need for data-centric approaches, improved topological evaluation, and greater dataset diversity to enable clinically robust and generalizable AI tools.
Towards contrast- and pathology-agnostic clinical fetal brain MRI segmentation using SynthSeg
Apr 14, 2025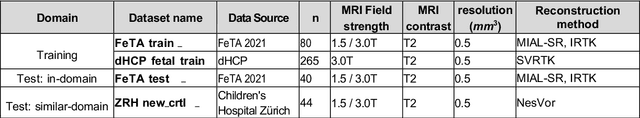



Abstract:Magnetic resonance imaging (MRI) has played a crucial role in fetal neurodevelopmental research. Structural annotations of MR images are an important step for quantitative analysis of the developing human brain, with Deep learning providing an automated alternative for this otherwise tedious manual process. However, segmentation performances of Convolutional Neural Networks often suffer from domain shift, where the network fails when applied to subjects that deviate from the distribution with which it is trained on. In this work, we aim to train networks capable of automatically segmenting fetal brain MRIs with a wide range of domain shifts pertaining to differences in subject physiology and acquisition environments, in particular shape-based differences commonly observed in pathological cases. We introduce a novel data-driven train-time sampling strategy that seeks to fully exploit the diversity of a given training dataset to enhance the domain generalizability of the trained networks. We adapted our sampler, together with other existing data augmentation techniques, to the SynthSeg framework, a generator that utilizes domain randomization to generate diverse training data, and ran thorough experimentations and ablation studies on a wide range of training/testing data to test the validity of the approaches. Our networks achieved notable improvements in the segmentation quality on testing subjects with intense anatomical abnormalities (p < 1e-4), though at the cost of a slighter decrease in performance in cases with fewer abnormalities. Our work also lays the foundation for future works on creating and adapting data-driven sampling strategies for other training pipelines.
Interpretable Embeddings for Segmentation-Free Single-Cell Analysis in Multiplex Imaging
Nov 02, 2024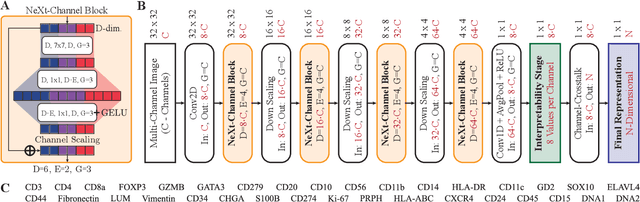

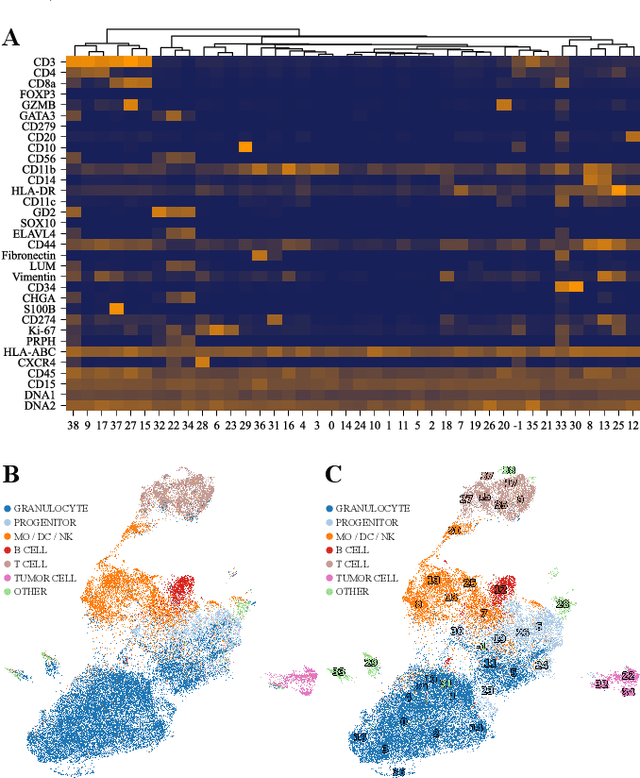
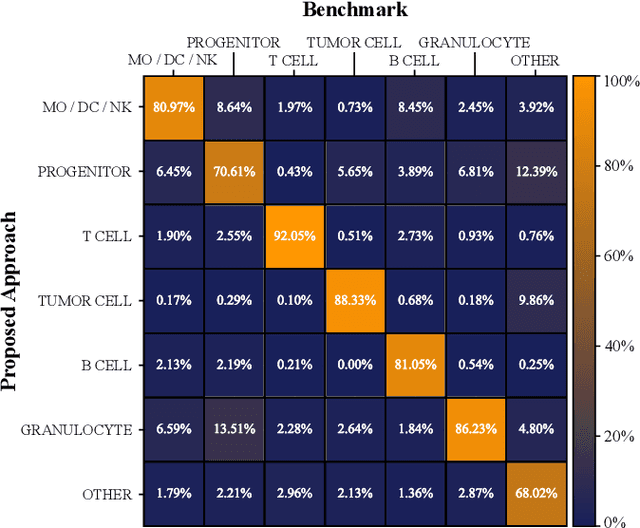
Abstract:Multiplex Imaging (MI) enables the simultaneous visualization of multiple biological markers in separate imaging channels at subcellular resolution, providing valuable insights into cell-type heterogeneity and spatial organization. However, current computational pipelines rely on cell segmentation algorithms, which require laborious fine-tuning and can introduce downstream errors due to inaccurate single-cell representations. We propose a segmentation-free deep learning approach that leverages grouped convolutions to learn interpretable embedded features from each imaging channel, enabling robust cell-type identification without manual feature selection. Validated on an Imaging Mass Cytometry dataset of 1.8 million cells from neuroblastoma patients, our method enables the accurate identification of known cell types, showcasing its scalability and suitability for high-dimensional MI data.
FISHing in Uncertainty: Synthetic Contrastive Learning for Genetic Aberration Detection
Nov 01, 2024Abstract:Detecting genetic aberrations is crucial in cancer diagnosis, typically through fluorescence in situ hybridization (FISH). However, existing FISH image classification methods face challenges due to signal variability, the need for costly manual annotations and fail to adequately address the intrinsic uncertainty. We introduce a novel approach that leverages synthetic images to eliminate the requirement for manual annotations and utilizes a joint contrastive and classification objective for training to account for inter-class variation effectively. We demonstrate the superior generalization capabilities and uncertainty calibration of our method, which is trained on synthetic data, by testing it on a manually annotated dataset of real-world FISH images. Our model offers superior calibration in terms of classification accuracy and uncertainty quantification with a classification accuracy of 96.7% among the 50% most certain cases. The presented end-to-end method reduces the demands on personnel and time and improves the diagnostic workflow due to its accuracy and adaptability. All code and data is publicly accessible at: https://github.com/SimonBon/FISHing
Multi-Center Fetal Brain Tissue Annotation (FeTA) Challenge 2022 Results
Feb 08, 2024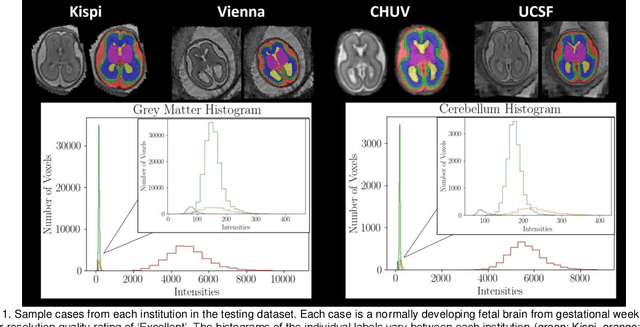
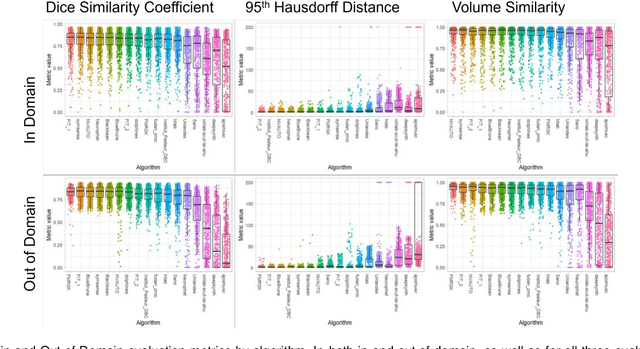
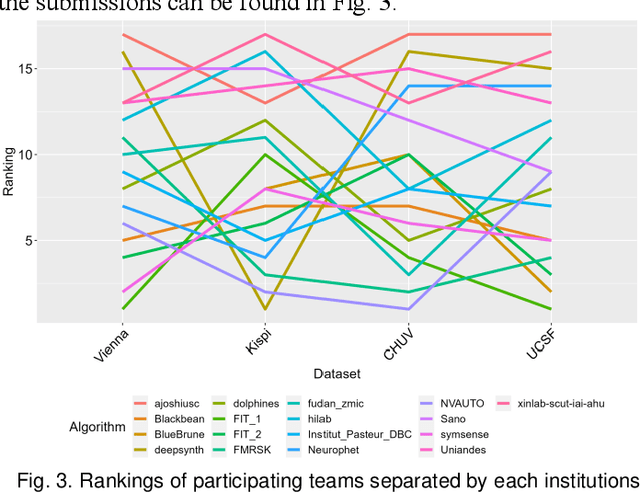
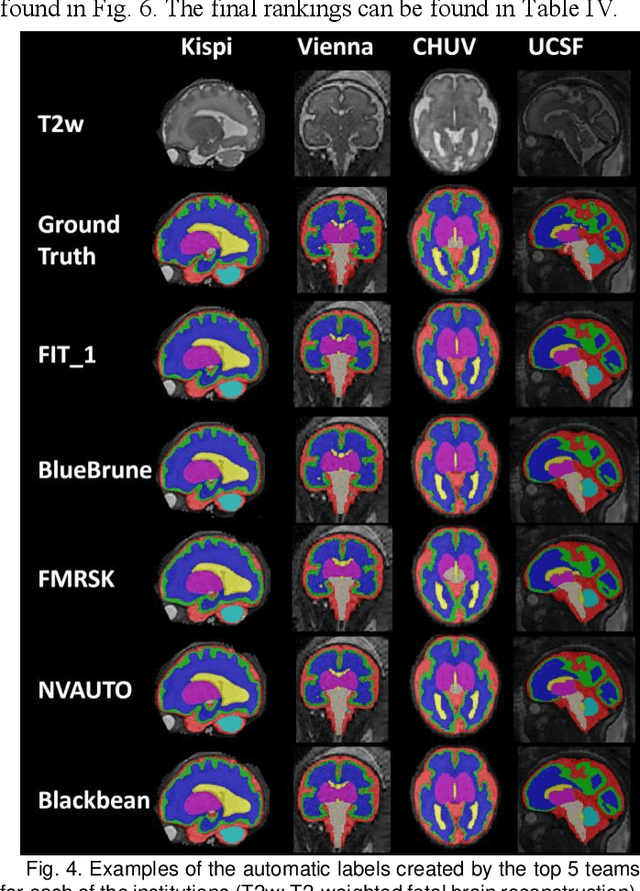
Abstract:Segmentation is a critical step in analyzing the developing human fetal brain. There have been vast improvements in automatic segmentation methods in the past several years, and the Fetal Brain Tissue Annotation (FeTA) Challenge 2021 helped to establish an excellent standard of fetal brain segmentation. However, FeTA 2021 was a single center study, and the generalizability of algorithms across different imaging centers remains unsolved, limiting real-world clinical applicability. The multi-center FeTA Challenge 2022 focuses on advancing the generalizability of fetal brain segmentation algorithms for magnetic resonance imaging (MRI). In FeTA 2022, the training dataset contained images and corresponding manually annotated multi-class labels from two imaging centers, and the testing data contained images from these two imaging centers as well as two additional unseen centers. The data from different centers varied in many aspects, including scanners used, imaging parameters, and fetal brain super-resolution algorithms applied. 16 teams participated in the challenge, and 17 algorithms were evaluated. Here, a detailed overview and analysis of the challenge results are provided, focusing on the generalizability of the submissions. Both in- and out of domain, the white matter and ventricles were segmented with the highest accuracy, while the most challenging structure remains the cerebral cortex due to anatomical complexity. The FeTA Challenge 2022 was able to successfully evaluate and advance generalizability of multi-class fetal brain tissue segmentation algorithms for MRI and it continues to benchmark new algorithms. The resulting new methods contribute to improving the analysis of brain development in utero.
Fetal Brain Tissue Annotation and Segmentation Challenge Results
Apr 20, 2022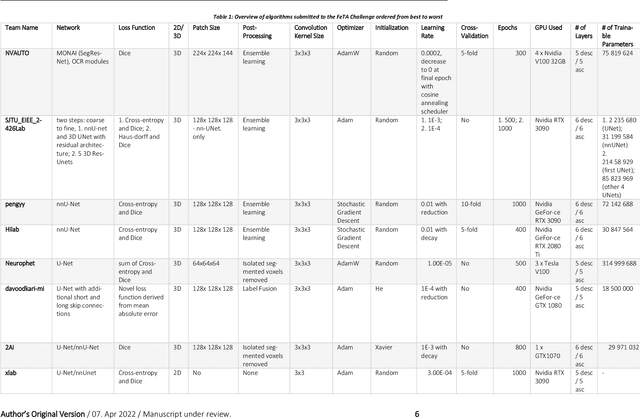
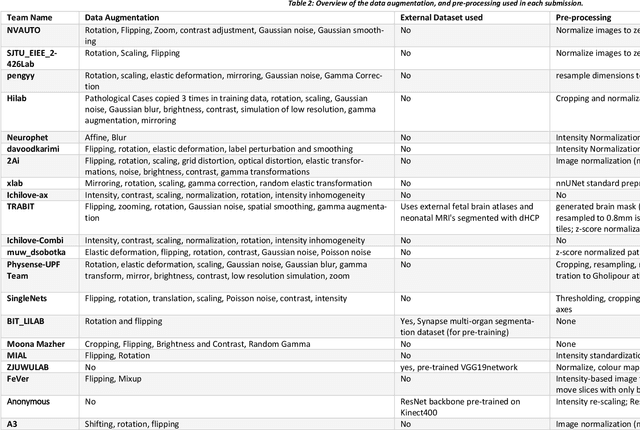
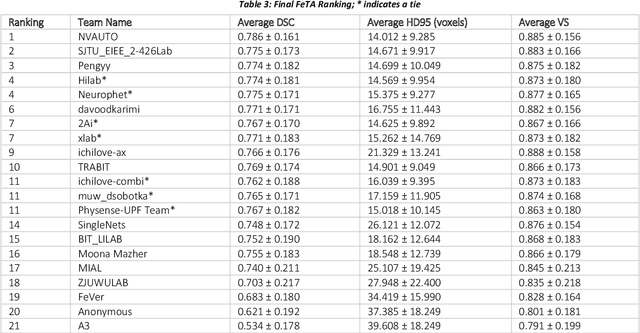
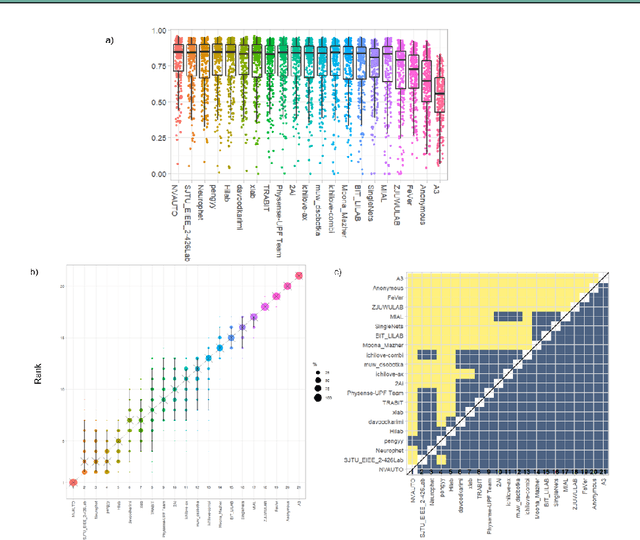
Abstract:In-utero fetal MRI is emerging as an important tool in the diagnosis and analysis of the developing human brain. Automatic segmentation of the developing fetal brain is a vital step in the quantitative analysis of prenatal neurodevelopment both in the research and clinical context. However, manual segmentation of cerebral structures is time-consuming and prone to error and inter-observer variability. Therefore, we organized the Fetal Tissue Annotation (FeTA) Challenge in 2021 in order to encourage the development of automatic segmentation algorithms on an international level. The challenge utilized FeTA Dataset, an open dataset of fetal brain MRI reconstructions segmented into seven different tissues (external cerebrospinal fluid, grey matter, white matter, ventricles, cerebellum, brainstem, deep grey matter). 20 international teams participated in this challenge, submitting a total of 21 algorithms for evaluation. In this paper, we provide a detailed analysis of the results from both a technical and clinical perspective. All participants relied on deep learning methods, mainly U-Nets, with some variability present in the network architecture, optimization, and image pre- and post-processing. The majority of teams used existing medical imaging deep learning frameworks. The main differences between the submissions were the fine tuning done during training, and the specific pre- and post-processing steps performed. The challenge results showed that almost all submissions performed similarly. Four of the top five teams used ensemble learning methods. However, one team's algorithm performed significantly superior to the other submissions, and consisted of an asymmetrical U-Net network architecture. This paper provides a first of its kind benchmark for future automatic multi-tissue segmentation algorithms for the developing human brain in utero.
Motion Correction and Volumetric Reconstruction for Fetal Functional Magnetic Resonance Imaging Data
Feb 11, 2022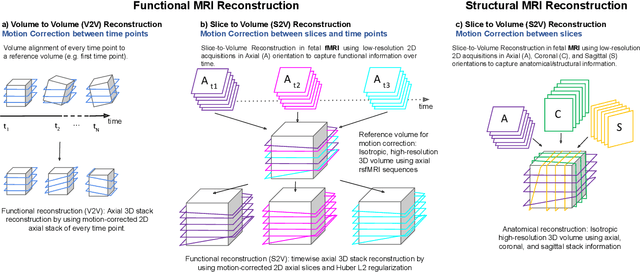
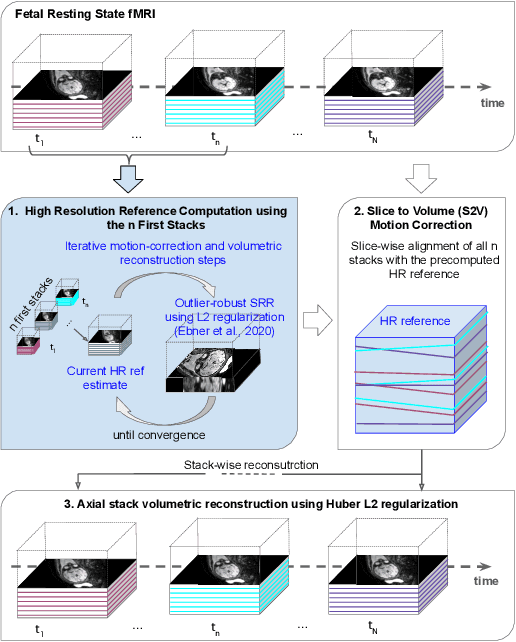
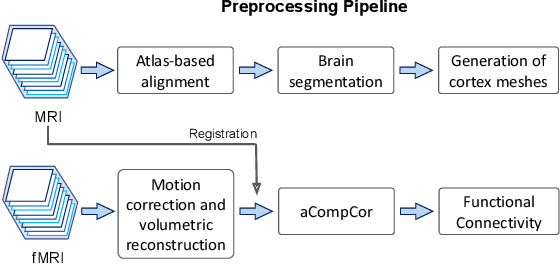
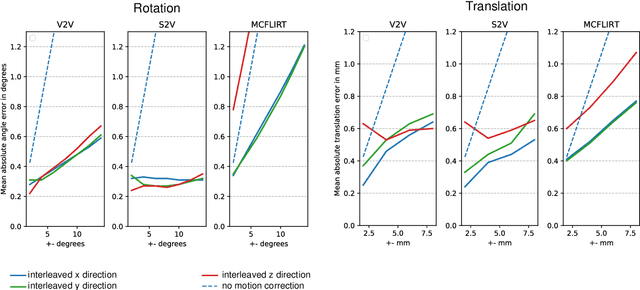
Abstract:Motion correction is an essential preprocessing step in functional Magnetic Resonance Imaging (fMRI) of the fetal brain with the aim to remove artifacts caused by fetal movement and maternal breathing and consequently to suppress erroneous signal correlations. Current motion correction approaches for fetal fMRI choose a single 3D volume from a specific acquisition timepoint with least motion artefacts as reference volume, and perform interpolation for the reconstruction of the motion corrected time series. The results can suffer, if no low-motion frame is available, and if reconstruction does not exploit any assumptions about the continuity of the fMRI signal. Here, we propose a novel framework, which estimates a high-resolution reference volume by using outlier-robust motion correction, and by utilizing Huber L2 regularization for intra-stack volumetric reconstruction of the motion-corrected fetal brain fMRI. We performed an extensive parameter study to investigate the effectiveness of motion estimation and present in this work benchmark metrics to quantify the effect of motion correction and regularised volumetric reconstruction approaches on functional connectivity computations. We demonstrate the proposed framework's ability to improve functional connectivity estimates, reproducibility and signal interpretability, which is clinically highly desirable for the establishment of prognostic noninvasive imaging biomarkers. The motion correction and volumetric reconstruction framework is made available as an open-source package of NiftyMIC.
4D iterative reconstruction of brain fMRI in the moving fetus
Nov 22, 2021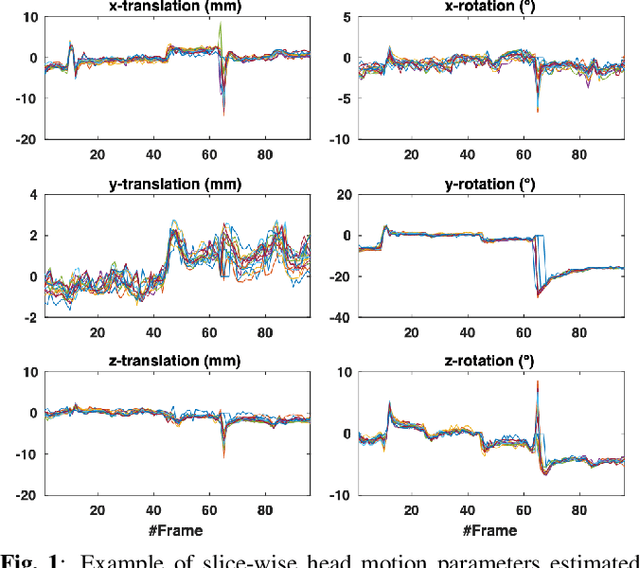
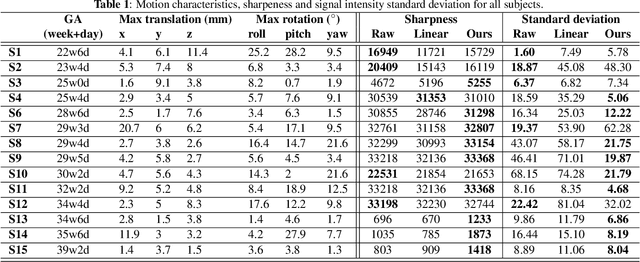
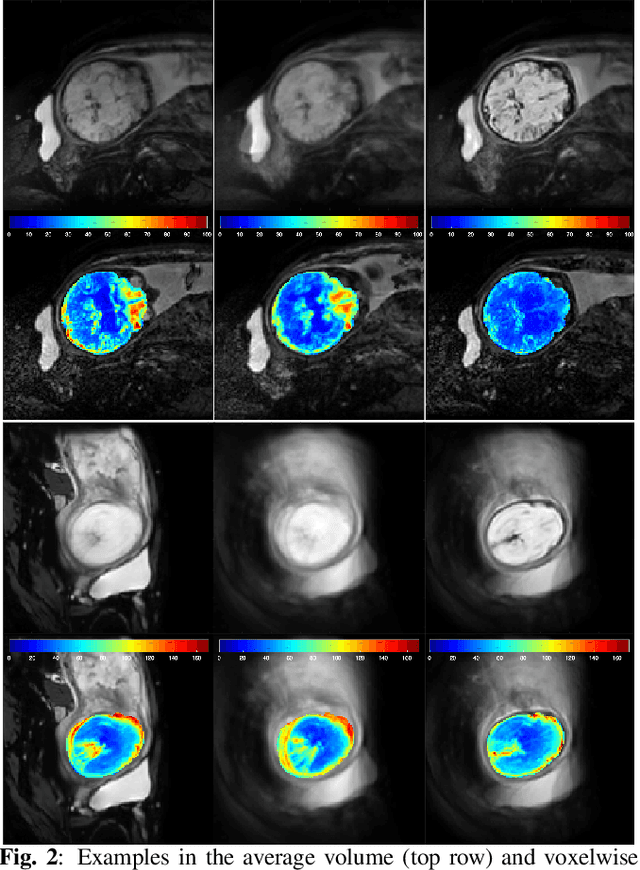
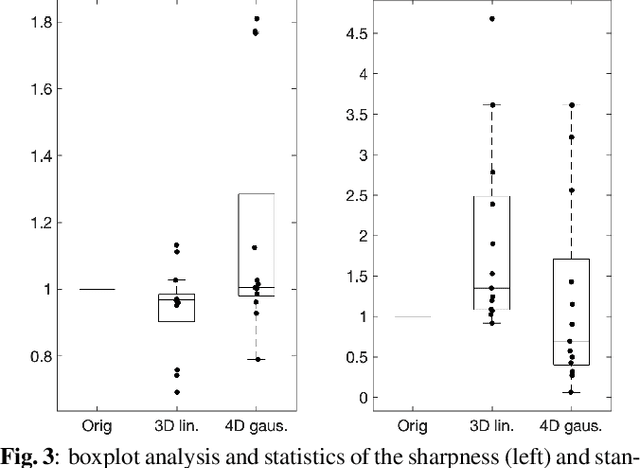
Abstract:Resting-state functional Magnetic Resonance Imaging (fMRI) is a powerful imaging technique for studying functional development of the brain in utero. However, unpredictable and excessive movement of fetuses has limited clinical application since it causes substantial signal fluctuations which can systematically alter observed patterns of functional connectivity. Previous studies have focused on the accurate estimation of the motion parameters in case of large fetal head movement and used a 3D single step interpolation approach at each timepoint to recover motion-free fMRI images. This does not guarantee that the reconstructed image corresponds to the minimum error representation of fMRI time series given the acquired data. Here, we propose a novel technique based on four dimensional iterative reconstruction of the scattered slices acquired during fetal fMRI. The accuracy of the proposed method was quantitatively evaluated on a group of real clinical fMRI fetuses. The results indicate improvements of reconstruction quality compared to the conventional 3D interpolation approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge