Long Bai
GeoLanG: Geometry-Aware Language-Guided Grasping with Unified RGB-D Multimodal Learning
Feb 04, 2026Abstract:Language-guided grasping has emerged as a promising paradigm for enabling robots to identify and manipulate target objects through natural language instructions, yet it remains highly challenging in cluttered or occluded scenes. Existing methods often rely on multi-stage pipelines that separate object perception and grasping, which leads to limited cross-modal fusion, redundant computation, and poor generalization in cluttered, occluded, or low-texture scenes. To address these limitations, we propose GeoLanG, an end-to-end multi-task framework built upon the CLIP architecture that unifies visual and linguistic inputs into a shared representation space for robust semantic alignment and improved generalization. To enhance target discrimination under occlusion and low-texture conditions, we explore a more effective use of depth information through the Depth-guided Geometric Module (DGGM), which converts depth into explicit geometric priors and injects them into the attention mechanism without additional computational overhead. In addition, we propose Adaptive Dense Channel Integration, which adaptively balances the contributions of multi-layer features to produce more discriminative and generalizable visual representations. Extensive experiments on the OCID-VLG dataset, as well as in both simulation and real-world hardware, demonstrate that GeoLanG enables precise and robust language-guided grasping in complex, cluttered environments, paving the way toward more reliable multimodal robotic manipulation in real-world human-centric settings.
Where It Moves, It Matters: Referring Surgical Instrument Segmentation via Motion
Jan 18, 2026Abstract:Enabling intuitive, language-driven interaction with surgical scenes is a critical step toward intelligent operating rooms and autonomous surgical robotic assistance. However, the task of referring segmentation, localizing surgical instruments based on natural language descriptions, remains underexplored in surgical videos, with existing approaches struggling to generalize due to reliance on static visual cues and predefined instrument names. In this work, we introduce SurgRef, a novel motion-guided framework that grounds free-form language expressions in instrument motion, capturing how tools move and interact across time, rather than what they look like. This allows models to understand and segment instruments even under occlusion, ambiguity, or unfamiliar terminology. To train and evaluate SurgRef, we present Ref-IMotion, a diverse, multi-institutional video dataset with dense spatiotemporal masks and rich motion-centric expressions. SurgRef achieves state-of-the-art accuracy and generalization across surgical procedures, setting a new benchmark for robust, language-driven surgical video segmentation.
Beyond Dialogue Time: Temporal Semantic Memory for Personalized LLM Agents
Jan 12, 2026Abstract:Memory enables Large Language Model (LLM) agents to perceive, store, and use information from past dialogues, which is essential for personalization. However, existing methods fail to properly model the temporal dimension of memory in two aspects: 1) Temporal inaccuracy: memories are organized by dialogue time rather than their actual occurrence time; 2) Temporal fragmentation: existing methods focus on point-wise memory, losing durative information that captures persistent states and evolving patterns. To address these limitations, we propose Temporal Semantic Memory (TSM), a memory framework that models semantic time for point-wise memory and supports the construction and utilization of durative memory. During memory construction, it first builds a semantic timeline rather than a dialogue one. Then, it consolidates temporally continuous and semantically related information into a durative memory. During memory utilization, it incorporates the query's temporal intent on the semantic timeline, enabling the retrieval of temporally appropriate durative memories and providing time-valid, duration-consistent context to support response generation. Experiments on LongMemEval and LoCoMo show that TSM consistently outperforms existing methods and achieves up to 12.2% absolute improvement in accuracy, demonstrating the effectiveness of the proposed method.
Maximizing Local Entropy Where It Matters: Prefix-Aware Localized LLM Unlearning
Jan 06, 2026Abstract:Machine unlearning aims to forget sensitive knowledge from Large Language Models (LLMs) while maintaining general utility. However, existing approaches typically treat all tokens in a response indiscriminately and enforce uncertainty over the entire vocabulary. This global treatment results in unnecessary utility degradation and extends optimization to content-agnostic regions. To address these limitations, we propose PALU (Prefix-Aware Localized Unlearning), a framework driven by a local entropy maximization objective across both temporal and vocabulary dimensions. PALU reveals that (i) suppressing the sensitive prefix alone is sufficient to sever the causal generation link, and (ii) flattening only the top-$k$ logits is adequate to maximize uncertainty in the critical subspace. These findings allow PALU to avoid redundant optimization across the full vocabulary and parameter space while minimizing collateral damage to general model performance. Extensive experiments validate that PALU achieves superior forgetting efficacy and utility preservation compared to state-of-the-art baselines.
More than Segmentation: Benchmarking SAM 3 for Segmentation, 3D Perception, and Reconstruction in Robotic Surgery
Dec 10, 2025Abstract:The recent SAM 3 and SAM 3D have introduced significant advancements over the predecessor, SAM 2, particularly with the integration of language-based segmentation and enhanced 3D perception capabilities. SAM 3 supports zero-shot segmentation across a wide range of prompts, including point, bounding box, and language-based prompts, allowing for more flexible and intuitive interactions with the model. In this empirical evaluation, we assess the performance of SAM 3 in robot-assisted surgery, benchmarking its zero-shot segmentation with point and bounding box prompts and exploring its effectiveness in dynamic video tracking, alongside its newly introduced language prompt segmentation. While language prompts show potential, their performance in the surgical domain is currently suboptimal, highlighting the need for further domain-specific training. Additionally, we investigate SAM 3D's depth reconstruction abilities, demonstrating its capacity to process surgical scene data and reconstruct 3D anatomical structures from 2D images. Through comprehensive testing on the MICCAI EndoVis 2017 and EndoVis 2018 benchmarks, SAM 3 shows clear improvements over SAM and SAM 2 in both image and video segmentation under spatial prompts, while the zero-shot evaluations of SAM 3D on SCARED, StereoMIS, and EndoNeRF indicate strong monocular depth estimation and realistic 3D instrument reconstruction, yet also reveal remaining limitations in complex, highly dynamic surgical scenes.
EndoIR: Degradation-Agnostic All-in-One Endoscopic Image Restoration via Noise-Aware Routing Diffusion
Nov 11, 2025Abstract:Endoscopic images often suffer from diverse and co-occurring degradations such as low lighting, smoke, and bleeding, which obscure critical clinical details. Existing restoration methods are typically task-specific and often require prior knowledge of the degradation type, limiting their robustness in real-world clinical use. We propose EndoIR, an all-in-one, degradation-agnostic diffusion-based framework that restores multiple degradation types using a single model. EndoIR introduces a Dual-Domain Prompter that extracts joint spatial-frequency features, coupled with an adaptive embedding that encodes both shared and task-specific cues as conditioning for denoising. To mitigate feature confusion in conventional concatenation-based conditioning, we design a Dual-Stream Diffusion architecture that processes clean and degraded inputs separately, with a Rectified Fusion Block integrating them in a structured, degradation-aware manner. Furthermore, Noise-Aware Routing Block improves efficiency by dynamically selecting only noise-relevant features during denoising. Experiments on SegSTRONG-C and CEC datasets demonstrate that EndoIR achieves state-of-the-art performance across multiple degradation scenarios while using fewer parameters than strong baselines, and downstream segmentation experiments confirm its clinical utility.
Comparative validation of surgical phase recognition, instrument keypoint estimation, and instrument instance segmentation in endoscopy: Results of the PhaKIR 2024 challenge
Jul 22, 2025Abstract:Reliable recognition and localization of surgical instruments in endoscopic video recordings are foundational for a wide range of applications in computer- and robot-assisted minimally invasive surgery (RAMIS), including surgical training, skill assessment, and autonomous assistance. However, robust performance under real-world conditions remains a significant challenge. Incorporating surgical context - such as the current procedural phase - has emerged as a promising strategy to improve robustness and interpretability. To address these challenges, we organized the Surgical Procedure Phase, Keypoint, and Instrument Recognition (PhaKIR) sub-challenge as part of the Endoscopic Vision (EndoVis) challenge at MICCAI 2024. We introduced a novel, multi-center dataset comprising thirteen full-length laparoscopic cholecystectomy videos collected from three distinct medical institutions, with unified annotations for three interrelated tasks: surgical phase recognition, instrument keypoint estimation, and instrument instance segmentation. Unlike existing datasets, ours enables joint investigation of instrument localization and procedural context within the same data while supporting the integration of temporal information across entire procedures. We report results and findings in accordance with the BIAS guidelines for biomedical image analysis challenges. The PhaKIR sub-challenge advances the field by providing a unique benchmark for developing temporally aware, context-driven methods in RAMIS and offers a high-quality resource to support future research in surgical scene understanding.
TR2M: Transferring Monocular Relative Depth to Metric Depth with Language Descriptions and Scale-Oriented Contrast
Jun 16, 2025Abstract:This work presents a generalizable framework to transfer relative depth to metric depth. Current monocular depth estimation methods are mainly divided into metric depth estimation (MMDE) and relative depth estimation (MRDE). MMDEs estimate depth in metric scale but are often limited to a specific domain. MRDEs generalize well across different domains, but with uncertain scales which hinders downstream applications. To this end, we aim to build up a framework to solve scale uncertainty and transfer relative depth to metric depth. Previous methods used language as input and estimated two factors for conducting rescaling. Our approach, TR2M, utilizes both text description and image as inputs and estimates two rescale maps to transfer relative depth to metric depth at pixel level. Features from two modalities are fused with a cross-modality attention module to better capture scale information. A strategy is designed to construct and filter confident pseudo metric depth for more comprehensive supervision. We also develop scale-oriented contrastive learning to utilize depth distribution as guidance to enforce the model learning about intrinsic knowledge aligning with the scale distribution. TR2M only exploits a small number of trainable parameters to train on datasets in various domains and experiments not only demonstrate TR2M's great performance in seen datasets but also reveal superior zero-shot capabilities on five unseen datasets. We show the huge potential in pixel-wise transferring relative depth to metric depth with language assistance. (Code is available at: https://github.com/BeileiCui/TR2M)
EndoARSS: Adapting Spatially-Aware Foundation Model for Efficient Activity Recognition and Semantic Segmentation in Endoscopic Surgery
Jun 07, 2025Abstract:Endoscopic surgery is the gold standard for robotic-assisted minimally invasive surgery, offering significant advantages in early disease detection and precise interventions. However, the complexity of surgical scenes, characterized by high variability in different surgical activity scenarios and confused image features between targets and the background, presents challenges for surgical environment understanding. Traditional deep learning models often struggle with cross-activity interference, leading to suboptimal performance in each downstream task. To address this limitation, we explore multi-task learning, which utilizes the interrelated features between tasks to enhance overall task performance. In this paper, we propose EndoARSS, a novel multi-task learning framework specifically designed for endoscopy surgery activity recognition and semantic segmentation. Built upon the DINOv2 foundation model, our approach integrates Low-Rank Adaptation to facilitate efficient fine-tuning while incorporating Task Efficient Shared Low-Rank Adapters to mitigate gradient conflicts across diverse tasks. Additionally, we introduce the Spatially-Aware Multi-Scale Attention that enhances feature representation discrimination by enabling cross-spatial learning of global information. In order to evaluate the effectiveness of our framework, we present three novel datasets, MTLESD, MTLEndovis and MTLEndovis-Gen, tailored for endoscopic surgery scenarios with detailed annotations for both activity recognition and semantic segmentation tasks. Extensive experiments demonstrate that EndoARSS achieves remarkable performance across multiple benchmarks, significantly improving both accuracy and robustness in comparison to existing models. These results underscore the potential of EndoARSS to advance AI-driven endoscopic surgical systems, offering valuable insights for enhancing surgical safety and efficiency.
KnowCoder-V2: Deep Knowledge Analysis
Jun 07, 2025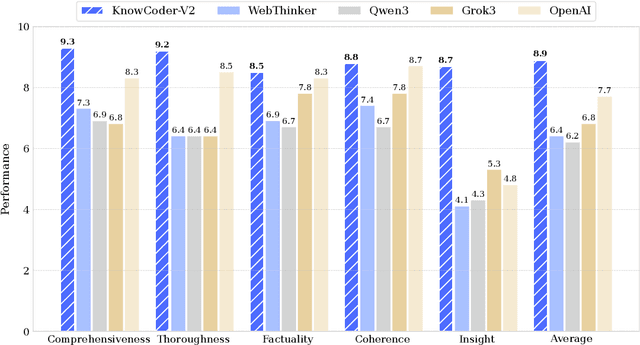
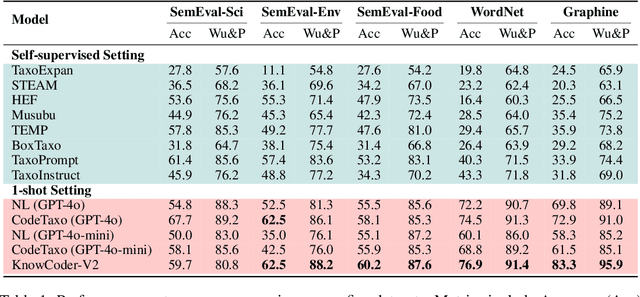
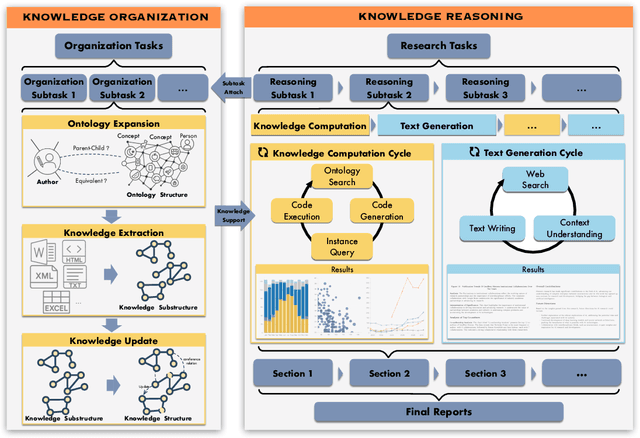
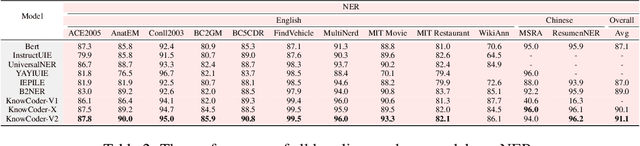
Abstract:Deep knowledge analysis tasks always involve the systematic extraction and association of knowledge from large volumes of data, followed by logical reasoning to discover insights. However, to solve such complex tasks, existing deep research frameworks face three major challenges: 1) They lack systematic organization and management of knowledge; 2) They operate purely online, making it inefficient for tasks that rely on shared and large-scale knowledge; 3) They cannot perform complex knowledge computation, limiting their abilities to produce insightful analytical results. Motivated by these, in this paper, we propose a \textbf{K}nowledgeable \textbf{D}eep \textbf{R}esearch (\textbf{KDR}) framework that empowers deep research with deep knowledge analysis capability. Specifically, it introduces an independent knowledge organization phase to preprocess large-scale, domain-relevant data into systematic knowledge offline. Based on this knowledge, it extends deep research with an additional kind of reasoning steps that perform complex knowledge computation in an online manner. To enhance the abilities of LLMs to solve knowledge analysis tasks in the above framework, we further introduce \textbf{\KCII}, an LLM that bridges knowledge organization and reasoning via unified code generation. For knowledge organization, it generates instantiation code for predefined classes, transforming data into knowledge objects. For knowledge computation, it generates analysis code and executes on the above knowledge objects to obtain deep analysis results. Experimental results on more than thirty datasets across six knowledge analysis tasks demonstrate the effectiveness of \KCII. Moreover, when integrated into the KDR framework, \KCII can generate high-quality reports with insightful analytical results compared to the mainstream deep research framework.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge