Mengya Xu
SurgWorld: Learning Surgical Robot Policies from Videos via World Modeling
Dec 30, 2025Abstract:Data scarcity remains a fundamental barrier to achieving fully autonomous surgical robots. While large scale vision language action (VLA) models have shown impressive generalization in household and industrial manipulation by leveraging paired video action data from diverse domains, surgical robotics suffers from the paucity of datasets that include both visual observations and accurate robot kinematics. In contrast, vast corpora of surgical videos exist, but they lack corresponding action labels, preventing direct application of imitation learning or VLA training. In this work, we aim to alleviate this problem by learning policy models from SurgWorld, a world model designed for surgical physical AI. We curated the Surgical Action Text Alignment (SATA) dataset with detailed action description specifically for surgical robots. Then we built SurgeWorld based on the most advanced physical AI world model and SATA. It's able to generate diverse, generalizable and realistic surgery videos. We are also the first to use an inverse dynamics model to infer pseudokinematics from synthetic surgical videos, producing synthetic paired video action data. We demonstrate that a surgical VLA policy trained with these augmented data significantly outperforms models trained only on real demonstrations on a real surgical robot platform. Our approach offers a scalable path toward autonomous surgical skill acquisition by leveraging the abundance of unlabeled surgical video and generative world modeling, thus opening the door to generalizable and data efficient surgical robot policies.
Comparative validation of surgical phase recognition, instrument keypoint estimation, and instrument instance segmentation in endoscopy: Results of the PhaKIR 2024 challenge
Jul 22, 2025Abstract:Reliable recognition and localization of surgical instruments in endoscopic video recordings are foundational for a wide range of applications in computer- and robot-assisted minimally invasive surgery (RAMIS), including surgical training, skill assessment, and autonomous assistance. However, robust performance under real-world conditions remains a significant challenge. Incorporating surgical context - such as the current procedural phase - has emerged as a promising strategy to improve robustness and interpretability. To address these challenges, we organized the Surgical Procedure Phase, Keypoint, and Instrument Recognition (PhaKIR) sub-challenge as part of the Endoscopic Vision (EndoVis) challenge at MICCAI 2024. We introduced a novel, multi-center dataset comprising thirteen full-length laparoscopic cholecystectomy videos collected from three distinct medical institutions, with unified annotations for three interrelated tasks: surgical phase recognition, instrument keypoint estimation, and instrument instance segmentation. Unlike existing datasets, ours enables joint investigation of instrument localization and procedural context within the same data while supporting the integration of temporal information across entire procedures. We report results and findings in accordance with the BIAS guidelines for biomedical image analysis challenges. The PhaKIR sub-challenge advances the field by providing a unique benchmark for developing temporally aware, context-driven methods in RAMIS and offers a high-quality resource to support future research in surgical scene understanding.
SAP-Bench: Benchmarking Multimodal Large Language Models in Surgical Action Planning
Jun 08, 2025Abstract:Effective evaluation is critical for driving advancements in MLLM research. The surgical action planning (SAP) task, which aims to generate future action sequences from visual inputs, demands precise and sophisticated analytical capabilities. Unlike mathematical reasoning, surgical decision-making operates in life-critical domains and requires meticulous, verifiable processes to ensure reliability and patient safety. This task demands the ability to distinguish between atomic visual actions and coordinate complex, long-horizon procedures, capabilities that are inadequately evaluated by current benchmarks. To address this gap, we introduce SAP-Bench, a large-scale, high-quality dataset designed to enable multimodal large language models (MLLMs) to perform interpretable surgical action planning. Our SAP-Bench benchmark, derived from the cholecystectomy procedures context with the mean duration of 1137.5s, and introduces temporally-grounded surgical action annotations, comprising the 1,226 clinically validated action clips (mean duration: 68.7s) capturing five fundamental surgical actions across 74 procedures. The dataset provides 1,152 strategically sampled current frames, each paired with the corresponding next action as multimodal analysis anchors. We propose the MLLM-SAP framework that leverages MLLMs to generate next action recommendations from the current surgical scene and natural language instructions, enhanced with injected surgical domain knowledge. To assess our dataset's effectiveness and the broader capabilities of current models, we evaluate seven state-of-the-art MLLMs (e.g., OpenAI-o1, GPT-4o, QwenVL2.5-72B, Claude-3.5-Sonnet, GeminiPro2.5, Step-1o, and GLM-4v) and reveal critical gaps in next action prediction performance.
EndoARSS: Adapting Spatially-Aware Foundation Model for Efficient Activity Recognition and Semantic Segmentation in Endoscopic Surgery
Jun 07, 2025Abstract:Endoscopic surgery is the gold standard for robotic-assisted minimally invasive surgery, offering significant advantages in early disease detection and precise interventions. However, the complexity of surgical scenes, characterized by high variability in different surgical activity scenarios and confused image features between targets and the background, presents challenges for surgical environment understanding. Traditional deep learning models often struggle with cross-activity interference, leading to suboptimal performance in each downstream task. To address this limitation, we explore multi-task learning, which utilizes the interrelated features between tasks to enhance overall task performance. In this paper, we propose EndoARSS, a novel multi-task learning framework specifically designed for endoscopy surgery activity recognition and semantic segmentation. Built upon the DINOv2 foundation model, our approach integrates Low-Rank Adaptation to facilitate efficient fine-tuning while incorporating Task Efficient Shared Low-Rank Adapters to mitigate gradient conflicts across diverse tasks. Additionally, we introduce the Spatially-Aware Multi-Scale Attention that enhances feature representation discrimination by enabling cross-spatial learning of global information. In order to evaluate the effectiveness of our framework, we present three novel datasets, MTLESD, MTLEndovis and MTLEndovis-Gen, tailored for endoscopic surgery scenarios with detailed annotations for both activity recognition and semantic segmentation tasks. Extensive experiments demonstrate that EndoARSS achieves remarkable performance across multiple benchmarks, significantly improving both accuracy and robustness in comparison to existing models. These results underscore the potential of EndoARSS to advance AI-driven endoscopic surgical systems, offering valuable insights for enhancing surgical safety and efficiency.
ETSM: Automating Dissection Trajectory Suggestion and Confidence Map-Based Safety Margin Prediction for Robot-assisted Endoscopic Submucosal Dissection
Nov 28, 2024Abstract:Robot-assisted Endoscopic Submucosal Dissection (ESD) improves the surgical procedure by providing a more comprehensive view through advanced robotic instruments and bimanual operation, thereby enhancing dissection efficiency and accuracy. Accurate prediction of dissection trajectories is crucial for better decision-making, reducing intraoperative errors, and improving surgical training. Nevertheless, predicting these trajectories is challenging due to variable tumor margins and dynamic visual conditions. To address this issue, we create the ESD Trajectory and Confidence Map-based Safety Margin (ETSM) dataset with $1849$ short clips, focusing on submucosal dissection with a dual-arm robotic system. We also introduce a framework that combines optimal dissection trajectory prediction with a confidence map-based safety margin, providing a more secure and intelligent decision-making tool to minimize surgical risks for ESD procedures. Additionally, we propose the Regression-based Confidence Map Prediction Network (RCMNet), which utilizes a regression approach to predict confidence maps for dissection areas, thereby delineating various levels of safety margins. We evaluate our RCMNet using three distinct experimental setups: in-domain evaluation, robustness assessment, and out-of-domain evaluation. Experimental results show that our approach excels in the confidence map-based safety margin prediction task, achieving a mean absolute error (MAE) of only $3.18$. To the best of our knowledge, this is the first study to apply a regression approach for visual guidance concerning delineating varying safety levels of dissection areas. Our approach bridges gaps in current research by improving prediction accuracy and enhancing the safety of the dissection process, showing great clinical significance in practice.
PDZSeg: Adapting the Foundation Model for Dissection Zone Segmentation with Visual Prompts in Robot-assisted Endoscopic Submucosal Dissection
Nov 27, 2024Abstract:Purpose: Endoscopic surgical environments present challenges for dissection zone segmentation due to unclear boundaries between tissue types, leading to segmentation errors where models misidentify or overlook edges. This study aims to provide precise dissection zone suggestions during endoscopic submucosal dissection (ESD) procedures, enhancing ESD safety. Methods: We propose the Prompted-based Dissection Zone Segmentation (PDZSeg) model, designed to leverage diverse visual prompts such as scribbles and bounding boxes. By overlaying these prompts onto images and fine-tuning a foundational model on a specialized dataset, our approach improves segmentation performance and user experience through flexible input methods. Results: The PDZSeg model was validated using three experimental setups: in-domain evaluation, variability in visual prompt availability, and robustness assessment. Using the ESD-DZSeg dataset, results show that our method outperforms state-of-the-art segmentation approaches. This is the first study to integrate visual prompt design into dissection zone segmentation. Conclusion: The PDZSeg model effectively utilizes visual prompts to enhance segmentation performance and user experience, supported by the novel ESD-DZSeg dataset as a benchmark for dissection zone segmentation in ESD. Our work establishes a foundation for future research.
Benchmarking Robustness of Endoscopic Depth Estimation with Synthetically Corrupted Data
Sep 24, 2024Abstract:Accurate depth perception is crucial for patient outcomes in endoscopic surgery, yet it is compromised by image distortions common in surgical settings. To tackle this issue, our study presents a benchmark for assessing the robustness of endoscopic depth estimation models. We have compiled a comprehensive dataset that reflects real-world conditions, incorporating a range of synthetically induced corruptions at varying severity levels. To further this effort, we introduce the Depth Estimation Robustness Score (DERS), a novel metric that combines measures of error, accuracy, and robustness to meet the multifaceted requirements of surgical applications. This metric acts as a foundational element for evaluating performance, establishing a new paradigm for the comparative analysis of depth estimation technologies. Additionally, we set forth a benchmark focused on robustness for the evaluation of depth estimation in endoscopic surgery, with the aim of driving progress in model refinement. A thorough analysis of two monocular depth estimation models using our framework reveals crucial information about their reliability under adverse conditions. Our results emphasize the essential need for algorithms that can tolerate data corruption, thereby advancing discussions on improving model robustness. The impact of this research transcends theoretical frameworks, providing concrete gains in surgical precision and patient safety. This study establishes a benchmark for the robustness of depth estimation and serves as a foundation for developing more resilient surgical support technologies. Code is available at https://github.com/lofrienger/EndoDepthBenchmark.
SAM 2 in Robotic Surgery: An Empirical Evaluation for Robustness and Generalization in Surgical Video Segmentation
Aug 08, 2024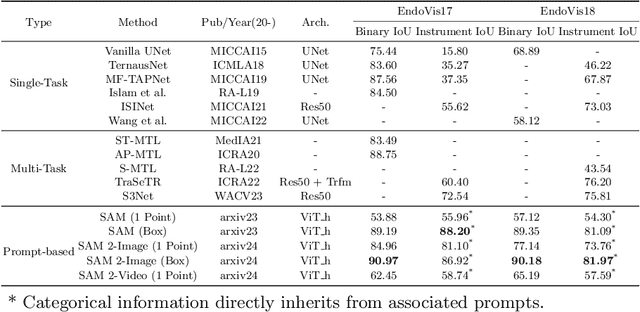



Abstract:The recent Segment Anything Model (SAM) 2 has demonstrated remarkable foundational competence in semantic segmentation, with its memory mechanism and mask decoder further addressing challenges in video tracking and object occlusion, thereby achieving superior results in interactive segmentation for both images and videos. Building upon our previous empirical studies, we further explore the zero-shot segmentation performance of SAM 2 in robot-assisted surgery based on prompts, alongside its robustness against real-world corruption. For static images, we employ two forms of prompts: 1-point and bounding box, while for video sequences, the 1-point prompt is applied to the initial frame. Through extensive experimentation on the MICCAI EndoVis 2017 and EndoVis 2018 benchmarks, SAM 2, when utilizing bounding box prompts, outperforms state-of-the-art (SOTA) methods in comparative evaluations. The results with point prompts also exhibit a substantial enhancement over SAM's capabilities, nearing or even surpassing existing unprompted SOTA methodologies. Besides, SAM 2 demonstrates improved inference speed and less performance degradation against various image corruption. Although slightly unsatisfactory results remain in specific edges or regions, SAM 2's robust adaptability to 1-point prompts underscores its potential for downstream surgical tasks with limited prompt requirements.
A Review of 3D Reconstruction Techniques for Deformable Tissues in Robotic Surgery
Aug 08, 2024Abstract:As a crucial and intricate task in robotic minimally invasive surgery, reconstructing surgical scenes using stereo or monocular endoscopic video holds immense potential for clinical applications. NeRF-based techniques have recently garnered attention for the ability to reconstruct scenes implicitly. On the other hand, Gaussian splatting-based 3D-GS represents scenes explicitly using 3D Gaussians and projects them onto a 2D plane as a replacement for the complex volume rendering in NeRF. However, these methods face challenges regarding surgical scene reconstruction, such as slow inference, dynamic scenes, and surgical tool occlusion. This work explores and reviews state-of-the-art (SOTA) approaches, discussing their innovations and implementation principles. Furthermore, we replicate the models and conduct testing and evaluation on two datasets. The test results demonstrate that with advancements in these techniques, achieving real-time, high-quality reconstructions becomes feasible.
PitVQA: Image-grounded Text Embedding LLM for Visual Question Answering in Pituitary Surgery
May 22, 2024



Abstract:Visual Question Answering (VQA) within the surgical domain, utilizing Large Language Models (LLMs), offers a distinct opportunity to improve intra-operative decision-making and facilitate intuitive surgeon-AI interaction. However, the development of LLMs for surgical VQA is hindered by the scarcity of diverse and extensive datasets with complex reasoning tasks. Moreover, contextual fusion of the image and text modalities remains an open research challenge due to the inherent differences between these two types of information and the complexity involved in aligning them. This paper introduces PitVQA, a novel dataset specifically designed for VQA in endonasal pituitary surgery and PitVQA-Net, an adaptation of the GPT2 with a novel image-grounded text embedding for surgical VQA. PitVQA comprises 25 procedural videos and a rich collection of question-answer pairs spanning crucial surgical aspects such as phase and step recognition, context understanding, tool detection and localization, and tool-tissue interactions. PitVQA-Net consists of a novel image-grounded text embedding that projects image and text features into a shared embedding space and GPT2 Backbone with an excitation block classification head to generate contextually relevant answers within the complex domain of endonasal pituitary surgery. Our image-grounded text embedding leverages joint embedding, cross-attention and contextual representation to understand the contextual relationship between questions and surgical images. We demonstrate the effectiveness of PitVQA-Net on both the PitVQA and the publicly available EndoVis18-VQA dataset, achieving improvements in balanced accuracy of 8% and 9% over the most recent baselines, respectively. Our code and dataset is available at https://github.com/mobarakol/PitVQA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge