Fuhua Yan
MR Elastography with Optimization-Based Phase Unwrapping and Traveling Wave Expansion-based Neural Network (TWENN)
Jan 06, 2023



Abstract:Magnetic Resonance Elastography (MRE) can characterize biomechanical properties of soft tissue for disease diagnosis and treatment planning. However, complicated wavefields acquired from MRE coupled with noise pose challenges for accurate displacement extraction and modulus estimation. Here we propose a pipeline for processing MRE images using optimization-based displacement extraction and Traveling Wave Expansion-based Neural Network (TWENN) modulus estimation. Phase unwrapping and displacement extraction were achieved by optimization of an objective function with Dual Data Consistency (Dual-DC). A complex-valued neural network using displacement covariance as input has been constructed for the estimation of complex wavenumbers. A model of traveling wave expansion is used to generate training datasets with different levels of noise for the network. The complex shear modulus map is obtained by a fusion of multifrequency and multidirectional data. Validation using images of brain and liver simulation demonstrates the practical value of the proposed pipeline, which can estimate the biomechanical properties with minimum root-mean-square-errors compared with state-of-the-art methods. Applications of the proposed method for processing MRE images of phantom, brain, and liver show clear anatomical features and that the pipeline is robust to noise and has a good generalization capability.
Dual-Sampling Attention Network for Diagnosis of COVID-19 from Community Acquired Pneumonia
May 20, 2020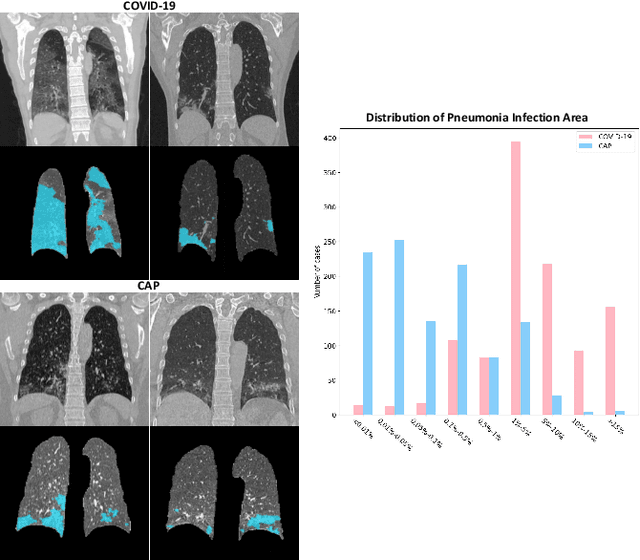
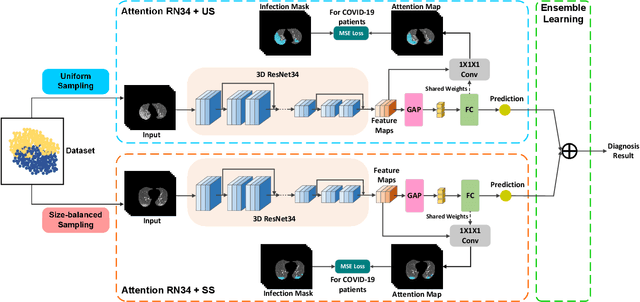
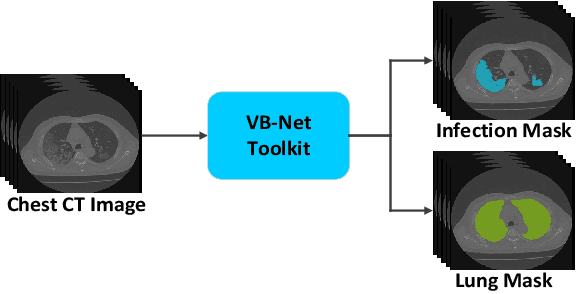
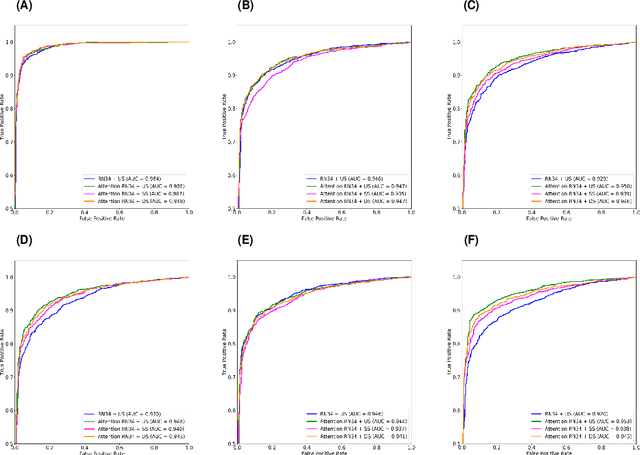
Abstract:The coronavirus disease (COVID-19) is rapidly spreading all over the world, and has infected more than 1,436,000 people in more than 200 countries and territories as of April 9, 2020. Detecting COVID-19 at early stage is essential to deliver proper healthcare to the patients and also to protect the uninfected population. To this end, we develop a dual-sampling attention network to automatically diagnose COVID- 19 from the community acquired pneumonia (CAP) in chest computed tomography (CT). In particular, we propose a novel online attention module with a 3D convolutional network (CNN) to focus on the infection regions in lungs when making decisions of diagnoses. Note that there exists imbalanced distribution of the sizes of the infection regions between COVID-19 and CAP, partially due to fast progress of COVID-19 after symptom onset. Therefore, we develop a dual-sampling strategy to mitigate the imbalanced learning. Our method is evaluated (to our best knowledge) upon the largest multi-center CT data for COVID-19 from 8 hospitals. In the training-validation stage, we collect 2186 CT scans from 1588 patients for a 5-fold cross-validation. In the testing stage, we employ another independent large-scale testing dataset including 2796 CT scans from 2057 patients. Results show that our algorithm can identify the COVID-19 images with the area under the receiver operating characteristic curve (AUC) value of 0.944, accuracy of 87.5%, sensitivity of 86.9%, specificity of 90.1%, and F1-score of 82.0%. With this performance, the proposed algorithm could potentially aid radiologists with COVID-19 diagnosis from CAP, especially in the early stage of the COVID-19 outbreak.
Hypergraph Learning for Identification of COVID-19 with CT Imaging
May 07, 2020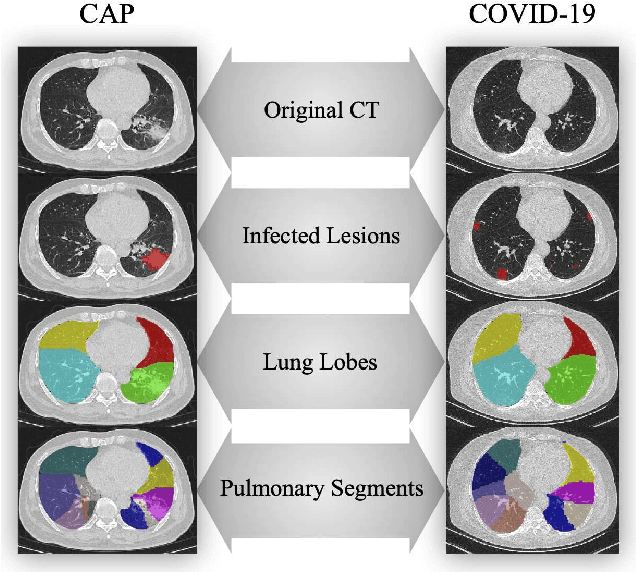

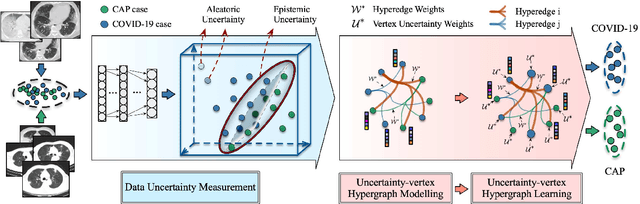
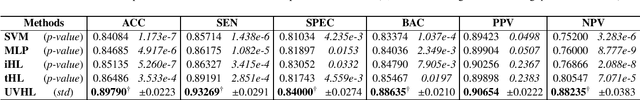
Abstract:The coronavirus disease, named COVID-19, has become the largest global public health crisis since it started in early 2020. CT imaging has been used as a complementary tool to assist early screening, especially for the rapid identification of COVID-19 cases from community acquired pneumonia (CAP) cases. The main challenge in early screening is how to model the confusing cases in the COVID-19 and CAP groups, with very similar clinical manifestations and imaging features. To tackle this challenge, we propose an Uncertainty Vertex-weighted Hypergraph Learning (UVHL) method to identify COVID-19 from CAP using CT images. In particular, multiple types of features (including regional features and radiomics features) are first extracted from CT image for each case. Then, the relationship among different cases is formulated by a hypergraph structure, with each case represented as a vertex in the hypergraph. The uncertainty of each vertex is further computed with an uncertainty score measurement and used as a weight in the hypergraph. Finally, a learning process of the vertex-weighted hypergraph is used to predict whether a new testing case belongs to COVID-19 or not. Experiments on a large multi-center pneumonia dataset, consisting of 2,148 COVID-19 cases and 1,182 CAP cases from five hospitals, are conducted to evaluate the performance of the proposed method. Results demonstrate the effectiveness and robustness of our proposed method on the identification of COVID-19 in comparison to state-of-the-art methods.
Adaptive Feature Selection Guided Deep Forest for COVID-19 Classification with Chest CT
May 07, 2020



Abstract:Chest computed tomography (CT) becomes an effective tool to assist the diagnosis of coronavirus disease-19 (COVID-19). Due to the outbreak of COVID-19 worldwide, using the computed-aided diagnosis technique for COVID-19 classification based on CT images could largely alleviate the burden of clinicians. In this paper, we propose an Adaptive Feature Selection guided Deep Forest (AFS-DF) for COVID-19 classification based on chest CT images. Specifically, we first extract location-specific features from CT images. Then, in order to capture the high-level representation of these features with the relatively small-scale data, we leverage a deep forest model to learn high-level representation of the features. Moreover, we propose a feature selection method based on the trained deep forest model to reduce the redundancy of features, where the feature selection could be adaptively incorporated with the COVID-19 classification model. We evaluated our proposed AFS-DF on COVID-19 dataset with 1495 patients of COVID-19 and 1027 patients of community acquired pneumonia (CAP). The accuracy (ACC), sensitivity (SEN), specificity (SPE) and AUC achieved by our method are 91.79%, 93.05%, 89.95% and 96.35%, respectively. Experimental results on the COVID-19 dataset suggest that the proposed AFS-DF achieves superior performance in COVID-19 vs. CAP classification, compared with 4 widely used machine learning methods.
Diagnosis of Coronavirus Disease 2019 (COVID-19) with Structured Latent Multi-View Representation Learning
May 06, 2020



Abstract:Recently, the outbreak of Coronavirus Disease 2019 (COVID-19) has spread rapidly across the world. Due to the large number of affected patients and heavy labor for doctors, computer-aided diagnosis with machine learning algorithm is urgently needed, and could largely reduce the efforts of clinicians and accelerate the diagnosis process. Chest computed tomography (CT) has been recognized as an informative tool for diagnosis of the disease. In this study, we propose to conduct the diagnosis of COVID-19 with a series of features extracted from CT images. To fully explore multiple features describing CT images from different views, a unified latent representation is learned which can completely encode information from different aspects of features and is endowed with promising class structure for separability. Specifically, the completeness is guaranteed with a group of backward neural networks (each for one type of features), while by using class labels the representation is enforced to be compact within COVID-19/community-acquired pneumonia (CAP) and also a large margin is guaranteed between different types of pneumonia. In this way, our model can well avoid overfitting compared to the case of directly projecting highdimensional features into classes. Extensive experimental results show that the proposed method outperforms all comparison methods, and rather stable performances are observed when varying the numbers of training data.
The Domain Shift Problem of Medical Image Segmentation and Vendor-Adaptation by Unet-GAN
Oct 30, 2019



Abstract:Convolutional neural network (CNN), in particular the Unet, is a powerful method for medical image segmentation. To date Unet has demonstrated state-of-art performance in many complex medical image segmentation tasks, especially under the condition when the training and testing data share the same distribution (i.e. come from the same source domain). However, in clinical practice, medical images are acquired from different vendors and centers. The performance of a U-Net trained from a particular source domain, when transferred to a different target domain (e.g. different vendor, acquisition parameter), can drop unexpectedly. Collecting a large amount of annotation from each new domain to retrain the U-Net is expensive, tedious, and practically impossible. In this work, we proposed a generic framework to address this problem, consisting of (1) an unpaired generative adversarial network (GAN) for vendor-adaptation, and (2) a Unet for object segmentation. In the proposed Unet-GAN architecture, GAN learns from Unet at the feature level that is segmentation-specific. We used cardiac cine MRI as the example, with three major vendors (Philips, Siemens, and GE) as three domains, while the methodology can be extended to medical images segmentation in general. The proposed method showed significant improvement of the segmentation results across vendors. The proposed Unet-GAN provides an annotation-free solution to the cross-vendor medical image segmentation problem, potentially extending a trained deep learning model to multi-center and multi-vendor use in real clinical scenario.
Learning-based Single-step Quantitative Susceptibility Mapping Reconstruction Without Brain Extraction
May 15, 2019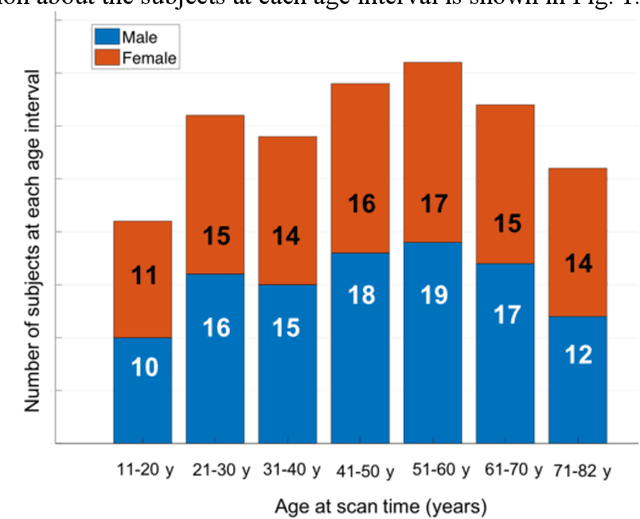
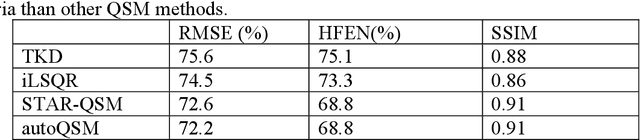
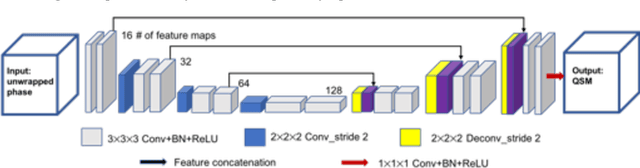
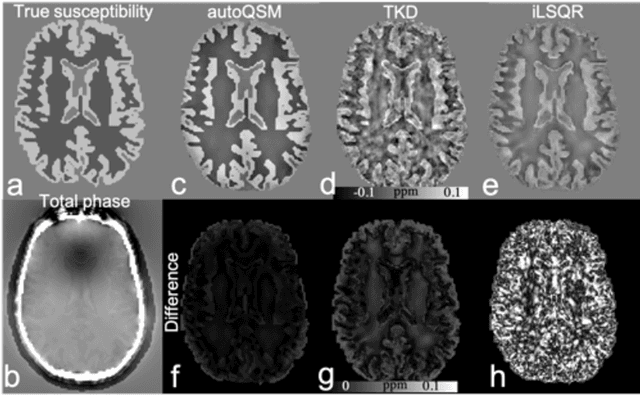
Abstract:Quantitative susceptibility mapping (QSM) estimates the underlying tissue magnetic susceptibility from MRI gradient-echo phase signal and typically requires several processing steps. These steps involve phase unwrapping, brain volume extraction, background phase removal and solving an ill-posed inverse problem. The resulting susceptibility map is known to suffer from inaccuracy near the edges of the brain tissues, in part due to imperfect brain extraction, edge erosion of the brain tissue and the lack of phase measurement outside the brain. This inaccuracy has thus hindered the application of QSM for measuring the susceptibility of tissues near the brain edges, e.g., quantifying cortical layers and generating superficial venography. To address these challenges, we propose a learning-based QSM reconstruction method that directly estimates the magnetic susceptibility from total phase images without the need for brain extraction and background phase removal, referred to as autoQSM. The neural network has a modified U-net structure and is trained using QSM maps computed by a two-step QSM method. 209 healthy subjects with ages ranging from 11 to 82 years were employed for patch-wise network training. The network was validated on data dissimilar to the training data, e.g. in vivo mouse brain data and brains with lesions, which suggests that the network has generalized and learned the underlying mathematical relationship between magnetic field perturbation and magnetic susceptibility. AutoQSM was able to recover magnetic susceptibility of anatomical structures near the edges of the brain including the veins covering the cortical surface, spinal cord and nerve tracts near the mouse brain boundaries. The advantages of high-quality maps, no need for brain volume extraction and high reconstruction speed demonstrate its potential for future applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge