Dijia Wu
A topology-preserving three-stage framework for fully-connected coronary artery extraction
Apr 02, 2025Abstract:Coronary artery extraction is a crucial prerequisite for computer-aided diagnosis of coronary artery disease. Accurately extracting the complete coronary tree remains challenging due to several factors, including presence of thin distal vessels, tortuous topological structures, and insufficient contrast. These issues often result in over-segmentation and under-segmentation in current segmentation methods. To address these challenges, we propose a topology-preserving three-stage framework for fully-connected coronary artery extraction. This framework includes vessel segmentation, centerline reconnection, and missing vessel reconstruction. First, we introduce a new centerline enhanced loss in the segmentation process. Second, for the broken vessel segments, we further propose a regularized walk algorithm to integrate distance, probabilities predicted by a centerline classifier, and directional cosine similarity, for reconnecting the centerlines. Third, we apply implicit neural representation and implicit modeling, to reconstruct the geometric model of the missing vessels. Experimental results show that our proposed framework outperforms existing methods, achieving Dice scores of 88.53\% and 85.07\%, with Hausdorff Distances (HD) of 1.07mm and 1.63mm on ASOCA and PDSCA datasets, respectively. Code will be available at https://github.com/YH-Qiu/CorSegRec.
Dual-Sampling Attention Network for Diagnosis of COVID-19 from Community Acquired Pneumonia
May 20, 2020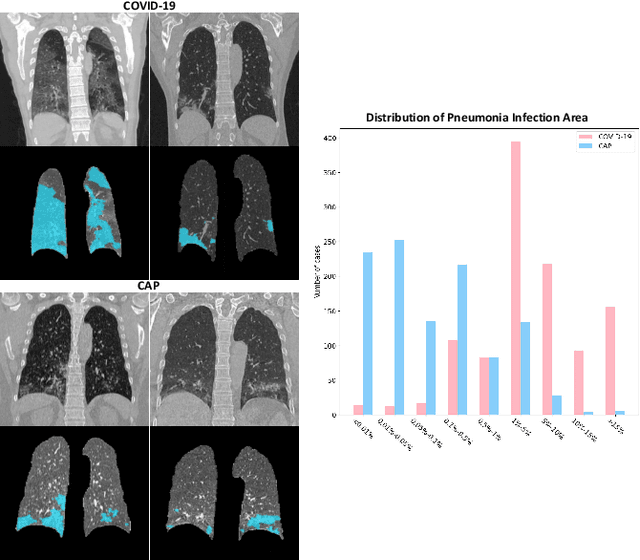
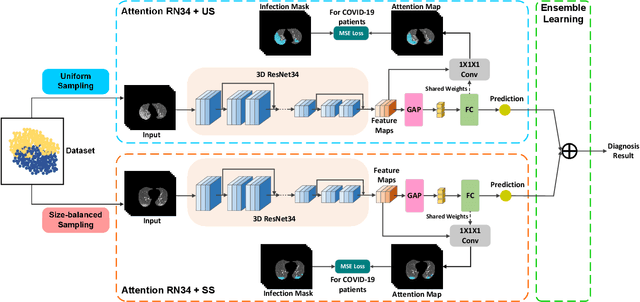
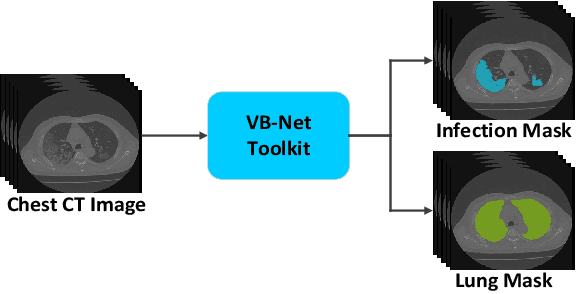
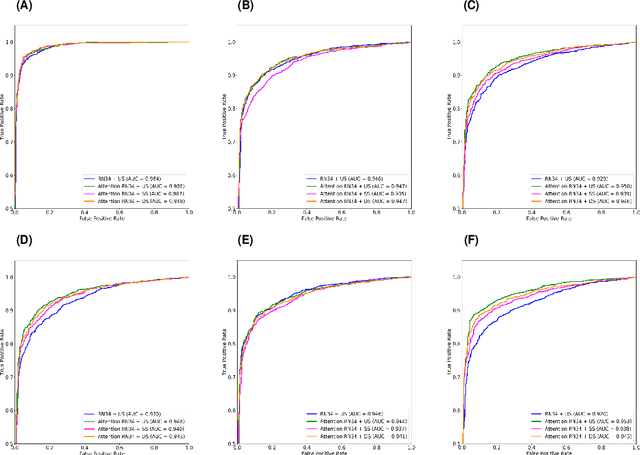
Abstract:The coronavirus disease (COVID-19) is rapidly spreading all over the world, and has infected more than 1,436,000 people in more than 200 countries and territories as of April 9, 2020. Detecting COVID-19 at early stage is essential to deliver proper healthcare to the patients and also to protect the uninfected population. To this end, we develop a dual-sampling attention network to automatically diagnose COVID- 19 from the community acquired pneumonia (CAP) in chest computed tomography (CT). In particular, we propose a novel online attention module with a 3D convolutional network (CNN) to focus on the infection regions in lungs when making decisions of diagnoses. Note that there exists imbalanced distribution of the sizes of the infection regions between COVID-19 and CAP, partially due to fast progress of COVID-19 after symptom onset. Therefore, we develop a dual-sampling strategy to mitigate the imbalanced learning. Our method is evaluated (to our best knowledge) upon the largest multi-center CT data for COVID-19 from 8 hospitals. In the training-validation stage, we collect 2186 CT scans from 1588 patients for a 5-fold cross-validation. In the testing stage, we employ another independent large-scale testing dataset including 2796 CT scans from 2057 patients. Results show that our algorithm can identify the COVID-19 images with the area under the receiver operating characteristic curve (AUC) value of 0.944, accuracy of 87.5%, sensitivity of 86.9%, specificity of 90.1%, and F1-score of 82.0%. With this performance, the proposed algorithm could potentially aid radiologists with COVID-19 diagnosis from CAP, especially in the early stage of the COVID-19 outbreak.
Adaptive Feature Selection Guided Deep Forest for COVID-19 Classification with Chest CT
May 07, 2020



Abstract:Chest computed tomography (CT) becomes an effective tool to assist the diagnosis of coronavirus disease-19 (COVID-19). Due to the outbreak of COVID-19 worldwide, using the computed-aided diagnosis technique for COVID-19 classification based on CT images could largely alleviate the burden of clinicians. In this paper, we propose an Adaptive Feature Selection guided Deep Forest (AFS-DF) for COVID-19 classification based on chest CT images. Specifically, we first extract location-specific features from CT images. Then, in order to capture the high-level representation of these features with the relatively small-scale data, we leverage a deep forest model to learn high-level representation of the features. Moreover, we propose a feature selection method based on the trained deep forest model to reduce the redundancy of features, where the feature selection could be adaptively incorporated with the COVID-19 classification model. We evaluated our proposed AFS-DF on COVID-19 dataset with 1495 patients of COVID-19 and 1027 patients of community acquired pneumonia (CAP). The accuracy (ACC), sensitivity (SEN), specificity (SPE) and AUC achieved by our method are 91.79%, 93.05%, 89.95% and 96.35%, respectively. Experimental results on the COVID-19 dataset suggest that the proposed AFS-DF achieves superior performance in COVID-19 vs. CAP classification, compared with 4 widely used machine learning methods.
Diagnosis of Coronavirus Disease 2019 (COVID-19) with Structured Latent Multi-View Representation Learning
May 06, 2020



Abstract:Recently, the outbreak of Coronavirus Disease 2019 (COVID-19) has spread rapidly across the world. Due to the large number of affected patients and heavy labor for doctors, computer-aided diagnosis with machine learning algorithm is urgently needed, and could largely reduce the efforts of clinicians and accelerate the diagnosis process. Chest computed tomography (CT) has been recognized as an informative tool for diagnosis of the disease. In this study, we propose to conduct the diagnosis of COVID-19 with a series of features extracted from CT images. To fully explore multiple features describing CT images from different views, a unified latent representation is learned which can completely encode information from different aspects of features and is endowed with promising class structure for separability. Specifically, the completeness is guaranteed with a group of backward neural networks (each for one type of features), while by using class labels the representation is enforced to be compact within COVID-19/community-acquired pneumonia (CAP) and also a large margin is guaranteed between different types of pneumonia. In this way, our model can well avoid overfitting compared to the case of directly projecting highdimensional features into classes. Extensive experimental results show that the proposed method outperforms all comparison methods, and rather stable performances are observed when varying the numbers of training data.
Large-Scale Screening of COVID-19 from Community Acquired Pneumonia using Infection Size-Aware Classification
Mar 22, 2020



Abstract:The worldwide spread of coronavirus disease (COVID-19) has become a threatening risk for global public health. It is of great importance to rapidly and accurately screen patients with COVID-19 from community acquired pneumonia (CAP). In this study, a total of 1658 patients with COVID-19 and 1027 patients of CAP underwent thin-section CT. All images were preprocessed to obtain the segmentations of both infections and lung fields, which were used to extract location-specific features. An infection Size Aware Random Forest method (iSARF) was proposed, in which subjects were automated categorized into groups with different ranges of infected lesion sizes, followed by random forests in each group for classification. Experimental results show that the proposed method yielded sensitivity of 0.907, specificity of 0.833, and accuracy of 0.879 under five-fold cross-validation. Large performance margins against comparison methods were achieved especially for the cases with infection size in the medium range, from 0.01% to 10%. The further inclusion of Radiomics features show slightly improvement. It is anticipated that our proposed framework could assist clinical decision making.
Semantic Context Forests for Learning-Based Knee Cartilage Segmentation in 3D MR Images
Apr 22, 2014
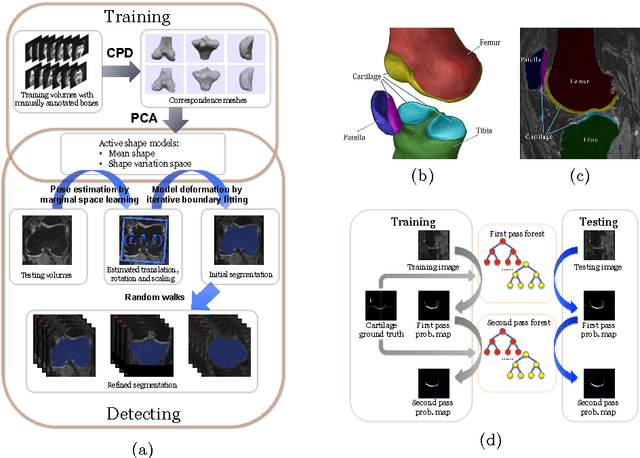
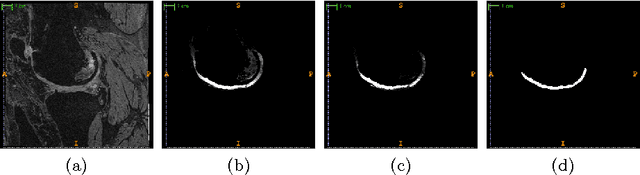
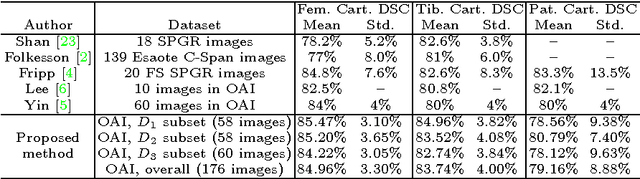
Abstract:The automatic segmentation of human knee cartilage from 3D MR images is a useful yet challenging task due to the thin sheet structure of the cartilage with diffuse boundaries and inhomogeneous intensities. In this paper, we present an iterative multi-class learning method to segment the femoral, tibial and patellar cartilage simultaneously, which effectively exploits the spatial contextual constraints between bone and cartilage, and also between different cartilages. First, based on the fact that the cartilage grows in only certain area of the corresponding bone surface, we extract the distance features of not only to the surface of the bone, but more informatively, to the densely registered anatomical landmarks on the bone surface. Second, we introduce a set of iterative discriminative classifiers that at each iteration, probability comparison features are constructed from the class confidence maps derived by previously learned classifiers. These features automatically embed the semantic context information between different cartilages of interest. Validated on a total of 176 volumes from the Osteoarthritis Initiative (OAI) dataset, the proposed approach demonstrates high robustness and accuracy of segmentation in comparison with existing state-of-the-art MR cartilage segmentation methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge