Ange Lou
From Preoperative CT to Postmastoidectomy Mesh Construction:1Mastoidectomy Shape Prediction for Cochlear Implant Surgery
Jan 07, 2026Abstract:Cochlear Implant (CI) surgery treats severe hearing loss by inserting an electrode array into the cochlea to stimulate the auditory nerve. An important step in this procedure is mastoidectomy, which removes part of the mastoid region of the temporal bone to provide surgical access. Accurate mastoidectomy shape prediction from preoperative imaging improves pre-surgical planning, reduces risks, and enhances surgical outcomes. Despite its importance, there are limited deep-learning-based studies regarding this topic due to the challenges of acquiring ground-truth labels. We address this gap by investigating self-supervised and weakly-supervised learning models to predict the mastoidectomy region without human annotations. We propose a hybrid self-supervised and weakly-supervised learning framework to predict the mastoidectomy region directly from preoperative CT scans, where the mastoid remains intact. Our hybrid method achieves a mean Dice score of 0.72 when predicting the complex and boundary-less mastoidectomy shape, surpassing state-of-the-art approaches and demonstrating strong performance. The method provides groundwork for constructing 3D postmastoidectomy surfaces directly from the corresponding preoperative CT scans. To our knowledge, this is the first work that integrating self-supervised and weakly-supervised learning for mastoidectomy shape prediction, offering a robust and efficient solution for CI surgical planning while leveraging 3D T-distribution loss in weakly-supervised medical imaging.
Collaborative Memory: Multi-User Memory Sharing in LLM Agents with Dynamic Access Control
May 23, 2025Abstract:Complex tasks are increasingly delegated to ensembles of specialized LLM-based agents that reason, communicate, and coordinate actions-both among themselves and through interactions with external tools, APIs, and databases. While persistent memory has been shown to enhance single-agent performance, most approaches assume a monolithic, single-user context-overlooking the benefits and challenges of knowledge transfer across users under dynamic, asymmetric permissions. We introduce Collaborative Memory, a framework for multi-user, multi-agent environments with asymmetric, time-evolving access controls encoded as bipartite graphs linking users, agents, and resources. Our system maintains two memory tiers: (1) private memory-private fragments visible only to their originating user; and (2) shared memory-selectively shared fragments. Each fragment carries immutable provenance attributes (contributing agents, accessed resources, and timestamps) to support retrospective permission checks. Granular read policies enforce current user-agent-resource constraints and project existing memory fragments into filtered transformed views. Write policies determine fragment retention and sharing, applying context-aware transformations to update the memory. Both policies may be designed conditioned on system, agent, and user-level information. Our framework enables safe, efficient, and interpretable cross-user knowledge sharing, with provable adherence to asymmetric, time-varying policies and full auditability of memory operations.
Neural Finite-State Machines for Surgical Phase Recognition
Nov 27, 2024


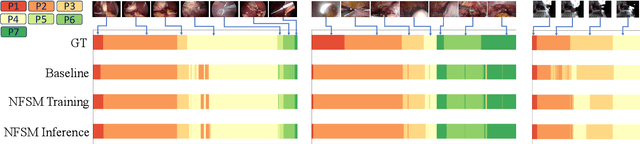
Abstract:Surgical phase recognition is essential for analyzing procedure-specific surgical videos. While recent transformer-based architectures have advanced sequence processing capabilities, they struggle with maintaining consistency across lengthy surgical procedures. Drawing inspiration from classical hidden Markov models' finite-state interpretations, we introduce the neural finite-state machine (NFSM) module, which bridges procedural understanding with deep learning approaches. NFSM combines procedure-level understanding with neural networks through global state embeddings, attention-based dynamic transition tables, and transition-aware training and inference mechanisms for offline and online applications. When integrated into our future-aware architecture, NFSM improves video-level accuracy, phase-level precision, recall, and Jaccard indices on Cholec80 datasets by 2.3, 3.2, 3.0, and 4.8 percentage points respectively. As an add-on module to existing state-of-the-art models like Surgformer, NFSM further enhances performance, demonstrating its complementary value. Extended experiments on non-surgical datasets validate NFSM's generalizability beyond surgical domains. Comprehensive experiments demonstrate that incorporating NSFM into deep learning frameworks enables more robust and consistent phase recognition across long procedural videos.
SynStitch: a Self-Supervised Learning Network for Ultrasound Image Stitching Using Synthetic Training Pairs and Indirect Supervision
Nov 11, 2024
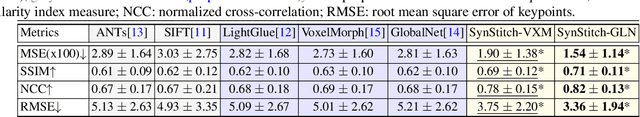
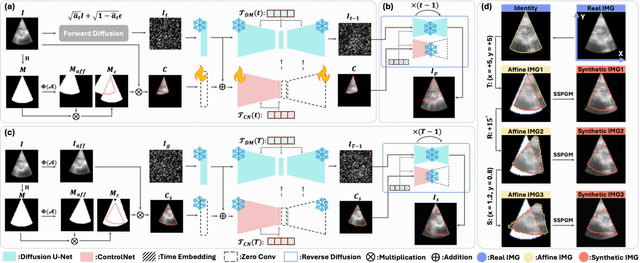

Abstract:Ultrasound (US) image stitching can expand the field-of-view (FOV) by combining multiple US images from varied probe positions. However, registering US images with only partially overlapping anatomical contents is a challenging task. In this work, we introduce SynStitch, a self-supervised framework designed for 2DUS stitching. SynStitch consists of a synthetic stitching pair generation module (SSPGM) and an image stitching module (ISM). SSPGM utilizes a patch-conditioned ControlNet to generate realistic 2DUS stitching pairs with known affine matrix from a single input image. ISM then utilizes this synthetic paired data to learn 2DUS stitching in a supervised manner. Our framework was evaluated against multiple leading methods on a kidney ultrasound dataset, demonstrating superior 2DUS stitching performance through both qualitative and quantitative analyses. The code will be made public upon acceptance of the paper.
DaRePlane: Direction-aware Representations for Dynamic Scene Reconstruction
Oct 18, 2024Abstract:Numerous recent approaches to modeling and re-rendering dynamic scenes leverage plane-based explicit representations, addressing slow training times associated with models like neural radiance fields (NeRF) and Gaussian splatting (GS). However, merely decomposing 4D dynamic scenes into multiple 2D plane-based representations is insufficient for high-fidelity re-rendering of scenes with complex motions. In response, we present DaRePlane, a novel direction-aware representation approach that captures scene dynamics from six different directions. This learned representation undergoes an inverse dual-tree complex wavelet transformation (DTCWT) to recover plane-based information. Within NeRF pipelines, DaRePlane computes features for each space-time point by fusing vectors from these recovered planes, then passed to a tiny MLP for color regression. When applied to Gaussian splatting, DaRePlane computes the features of Gaussian points, followed by a tiny multi-head MLP for spatial-time deformation prediction. Notably, to address redundancy introduced by the six real and six imaginary direction-aware wavelet coefficients, we introduce a trainable masking approach, mitigating storage issues without significant performance decline. To demonstrate the generality and efficiency of DaRePlane, we test it on both regular and surgical dynamic scenes, for both NeRF and GS systems. Extensive experiments show that DaRePlane yields state-of-the-art performance in novel view synthesis for various complex dynamic scenes.
Surgical Depth Anything: Depth Estimation for Surgical Scenes using Foundation Models
Oct 09, 2024


Abstract:Monocular depth estimation is crucial for tracking and reconstruction algorithms, particularly in the context of surgical videos. However, the inherent challenges in directly obtaining ground truth depth maps during surgery render supervised learning approaches impractical. While many self-supervised methods based on Structure from Motion (SfM) have shown promising results, they rely heavily on high-quality camera motion and require optimization on a per-patient basis. These limitations can be mitigated by leveraging the current state-of-the-art foundational model for depth estimation, Depth Anything. However, when directly applied to surgical scenes, Depth Anything struggles with issues such as blurring, bleeding, and reflections, resulting in suboptimal performance. This paper presents a fine-tuning of the Depth Anything model specifically for the surgical domain, aiming to deliver more accurate pixel-wise depth maps tailored to the unique requirements and challenges of surgical environments. Our fine-tuning approach significantly improves the model's performance in surgical scenes, reducing errors related to blurring and reflections, and achieving a more reliable and precise depth estimation.
NeuroBOLT: Resting-state EEG-to-fMRI Synthesis with Multi-dimensional Feature Mapping
Oct 07, 2024



Abstract:Functional magnetic resonance imaging (fMRI) is an indispensable tool in modern neuroscience, providing a non-invasive window into whole-brain dynamics at millimeter-scale spatial resolution. However, fMRI is constrained by issues such as high operation costs and immobility. With the rapid advancements in cross-modality synthesis and brain decoding, the use of deep neural networks has emerged as a promising solution for inferring whole-brain, high-resolution fMRI features directly from electroencephalography (EEG), a more widely accessible and portable neuroimaging modality. Nonetheless, the complex projection from neural activity to fMRI hemodynamic responses and the spatial ambiguity of EEG pose substantial challenges both in modeling and interpretability. Relatively few studies to date have developed approaches for EEG-fMRI translation, and although they have made significant strides, the inference of fMRI signals in a given study has been limited to a small set of brain areas and to a single condition (i.e., either resting-state or a specific task). The capability to predict fMRI signals in other brain areas, as well as to generalize across conditions, remain critical gaps in the field. To tackle these challenges, we introduce a novel and generalizable framework: NeuroBOLT, i.e., Neuro-to-BOLD Transformer, which leverages multi-dimensional representation learning from temporal, spatial, and spectral domains to translate raw EEG data to the corresponding fMRI activity signals across the brain. Our experiments demonstrate that NeuroBOLT effectively reconstructs resting-state fMRI signals from primary sensory, high-level cognitive areas, and deep subcortical brain regions, achieving state-of-the-art accuracy and significantly advancing the integration of these two modalities.
Zero-Shot Surgical Tool Segmentation in Monocular Video Using Segment Anything Model 2
Aug 03, 2024Abstract:The Segment Anything Model 2 (SAM 2) is the latest generation foundation model for image and video segmentation. Trained on the expansive Segment Anything Video (SA-V) dataset, which comprises 35.5 million masks across 50.9K videos, SAM 2 advances its predecessor's capabilities by supporting zero-shot segmentation through various prompts (e.g., points, boxes, and masks). Its robust zero-shot performance and efficient memory usage make SAM 2 particularly appealing for surgical tool segmentation in videos, especially given the scarcity of labeled data and the diversity of surgical procedures. In this study, we evaluate the zero-shot video segmentation performance of the SAM 2 model across different types of surgeries, including endoscopy and microscopy. We also assess its performance on videos featuring single and multiple tools of varying lengths to demonstrate SAM 2's applicability and effectiveness in the surgical domain. We found that: 1) SAM 2 demonstrates a strong capability for segmenting various surgical videos; 2) When new tools enter the scene, additional prompts are necessary to maintain segmentation accuracy; and 3) Specific challenges inherent to surgical videos can impact the robustness of SAM 2.
Divide and Fuse: Body Part Mesh Recovery from Partially Visible Human Images
Jul 12, 2024



Abstract:We introduce a novel bottom-up approach for human body mesh reconstruction, specifically designed to address the challenges posed by partial visibility and occlusion in input images. Traditional top-down methods, relying on whole-body parametric models like SMPL, falter when only a small part of the human is visible, as they require visibility of most of the human body for accurate mesh reconstruction. To overcome this limitation, our method employs a "Divide and Fuse (D&F)" strategy, reconstructing human body parts independently before fusing them, thereby ensuring robustness against occlusions. We design Human Part Parametric Models (HPPM) that independently reconstruct the mesh from a few shape and global-location parameters, without inter-part dependency. A specially designed fusion module then seamlessly integrates the reconstructed parts, even when only a few are visible. We harness a large volume of ground-truth SMPL data to train our parametric mesh models. To facilitate the training and evaluation of our method, we have established benchmark datasets featuring images of partially visible humans with HPPM annotations. Our experiments, conducted on these benchmark datasets, demonstrate the effectiveness of our D&F method, particularly in scenarios with substantial invisibility, where traditional approaches struggle to maintain reconstruction quality.
Monocular Microscope to CT Registration using Pose Estimation of the Incus for Augmented Reality Cochlear Implant Surgery
Mar 12, 2024Abstract:For those experiencing severe-to-profound sensorineural hearing loss, the cochlear implant (CI) is the preferred treatment. Augmented reality (AR) aided surgery can potentially improve CI procedures and hearing outcomes. Typically, AR solutions for image-guided surgery rely on optical tracking systems to register pre-operative planning information to the display so that hidden anatomy or other important information can be overlayed and co-registered with the view of the surgical scene. In this paper, our goal is to develop a method that permits direct 2D-to-3D registration of the microscope video to the pre-operative Computed Tomography (CT) scan without the need for external tracking equipment. Our proposed solution involves using surface mapping of a portion of the incus in surgical recordings and determining the pose of this structure relative to the surgical microscope by performing pose estimation via the perspective-n-point (PnP) algorithm. This registration can then be applied to pre-operative segmentations of other anatomy-of-interest, as well as the planned electrode insertion trajectory to co-register this information for the AR display. Our results demonstrate the accuracy with an average rotation error of less than 25 degrees and a translation error of less than 2 mm, 3 mm, and 0.55% for the x, y, and z axes, respectively. Our proposed method has the potential to be applicable and generalized to other surgical procedures while only needing a monocular microscope during intra-operation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge