Luyuan Xie
FedSRD: Sparsify-Reconstruct-Decompose for Communication-Efficient Federated Large Language Models Fine-Tuning
Oct 06, 2025



Abstract:The current paradigm of training large language models (LLMs) on publicly available Web data is becoming unsustainable, with high-quality data sources in specialized domains nearing exhaustion. Federated Learning (FL) emerges as a practical solution for the next generation of AI on a decentralized Web, enabling privacy-preserving collaborative fine-tuning by leveraging private data distributed across a global client base. While Low-Rank Adaptation (LoRA) is the standard for efficient fine-tuning, its application in federated settings presents a critical challenge: communication overhead remains a significant bottleneck across the Web's heterogeneous network conditions. The structural redundancy within LoRA parameters not only incurs a heavy communication burden but also introduces conflicts when aggregating client updates. To address this, we propose FedSRD, a Sparsify-Reconstruct-Decompose framework designed for communication-efficient FL. We first introduce an importance-aware sparsification method that preserves the structural integrity of LoRA updates to reduce the uploaded parameter count. The server then reconstructs and aggregates these updates in a full-rank space to mitigate conflicts. Finally, it decomposes the global update into a sparse low-rank format for broadcast, ensuring a symmetrically efficient cycle. We also propose an efficient variant, FedSRD-e, to reduce computational overhead. Experimental results on 10 benchmarks demonstrate that our framework significantly reduces communication costs by up to 90\% while even improving model performance on heterogeneous client data.
dFLMoE: Decentralized Federated Learning via Mixture of Experts for Medical Data Analysis
Mar 13, 2025Abstract:Federated learning has wide applications in the medical field. It enables knowledge sharing among different healthcare institutes while protecting patients' privacy. However, existing federated learning systems are typically centralized, requiring clients to upload client-specific knowledge to a central server for aggregation. This centralized approach would integrate the knowledge from each client into a centralized server, and the knowledge would be already undermined during the centralized integration before it reaches back to each client. Besides, the centralized approach also creates a dependency on the central server, which may affect training stability if the server malfunctions or connections are unstable. To address these issues, we propose a decentralized federated learning framework named dFLMoE. In our framework, clients directly exchange lightweight head models with each other. After exchanging, each client treats both local and received head models as individual experts, and utilizes a client-specific Mixture of Experts (MoE) approach to make collective decisions. This design not only reduces the knowledge damage with client-specific aggregations but also removes the dependency on the central server to enhance the robustness of the framework. We validate our framework on multiple medical tasks, demonstrating that our method evidently outperforms state-of-the-art approaches under both model homogeneity and heterogeneity settings.
FedVCK: Non-IID Robust and Communication-Efficient Federated Learning via Valuable Condensed Knowledge for Medical Image Analysis
Dec 24, 2024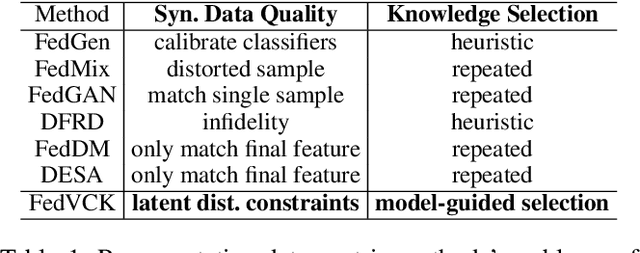
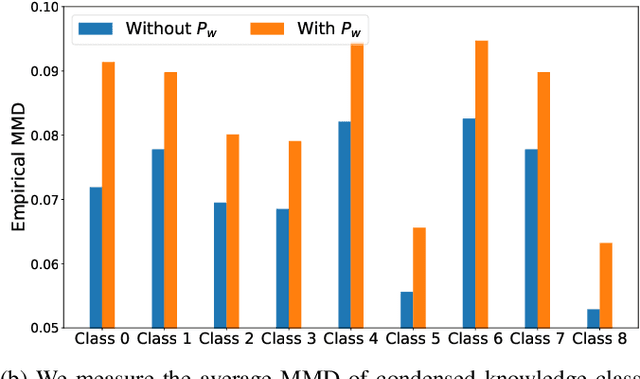
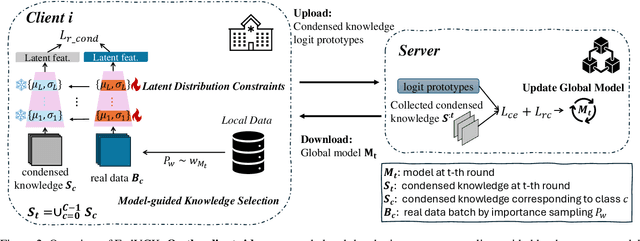
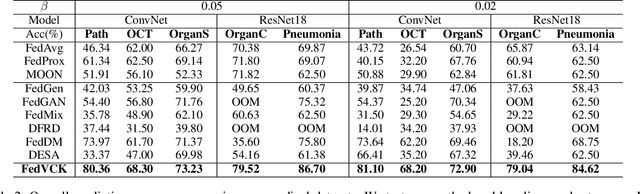
Abstract:Federated learning has become a promising solution for collaboration among medical institutions. However, data owned by each institution would be highly heterogeneous and the distribution is always non-independent and identical distribution (non-IID), resulting in client drift and unsatisfactory performance. Despite existing federated learning methods attempting to solve the non-IID problems, they still show marginal advantages but rely on frequent communication which would incur high costs and privacy concerns. In this paper, we propose a novel federated learning method: \textbf{Fed}erated learning via \textbf{V}aluable \textbf{C}ondensed \textbf{K}nowledge (FedVCK). We enhance the quality of condensed knowledge and select the most necessary knowledge guided by models, to tackle the non-IID problem within limited communication budgets effectively. Specifically, on the client side, we condense the knowledge of each client into a small dataset and further enhance the condensation procedure with latent distribution constraints, facilitating the effective capture of high-quality knowledge. During each round, we specifically target and condense knowledge that has not been assimilated by the current model, thereby preventing unnecessary repetition of homogeneous knowledge and minimizing the frequency of communications required. On the server side, we propose relational supervised contrastive learning to provide more supervision signals to aid the global model updating. Comprehensive experiments across various medical tasks show that FedVCK can outperform state-of-the-art methods, demonstrating that it's non-IID robust and communication-efficient.
Toward Personalized Federated Node Classification in One-shot Communication
Nov 18, 2024



Abstract:Federated Graph Learning (FGL) has become a promising paradigm for collaborative training with distributed and private graph data. One-shot Federated Learning (OFL) enables collaboration in a single communication round to largely reduce communication costs and potential security concerns. However, existing OFL methods are not designed for graph data and existing FGL methods are ineffective within one communication round under both data and model heterogeneity. To mitigate this gap, we are the first to propose a one-shot personalized federated graph learning method for node classification, which is also compatible with the Secure Aggregation scheme. We estimate and aggregate the statistics of class-wise feature distribution to generate a global pseudo-graph on the server, which could be used to train a global graph model. Furthermore, We reveal the under-explored problem of existing personalized FGL methods that their personalized models are biased and neglect the ability to generalize to minorities. To achieve better personalization and generalization simultaneously, we propose a two-stage personalized training to adaptively utilize the personal information from local data and global information from the global pseudo-graph. Comprehensive experiments on 8 multi-scale graph datasets under different partitions with various settings demonstrate our superior performance over state-of-the-art baselines.
Divide and Fuse: Body Part Mesh Recovery from Partially Visible Human Images
Jul 12, 2024



Abstract:We introduce a novel bottom-up approach for human body mesh reconstruction, specifically designed to address the challenges posed by partial visibility and occlusion in input images. Traditional top-down methods, relying on whole-body parametric models like SMPL, falter when only a small part of the human is visible, as they require visibility of most of the human body for accurate mesh reconstruction. To overcome this limitation, our method employs a "Divide and Fuse (D&F)" strategy, reconstructing human body parts independently before fusing them, thereby ensuring robustness against occlusions. We design Human Part Parametric Models (HPPM) that independently reconstruct the mesh from a few shape and global-location parameters, without inter-part dependency. A specially designed fusion module then seamlessly integrates the reconstructed parts, even when only a few are visible. We harness a large volume of ground-truth SMPL data to train our parametric mesh models. To facilitate the training and evaluation of our method, we have established benchmark datasets featuring images of partially visible humans with HPPM annotations. Our experiments, conducted on these benchmark datasets, demonstrate the effectiveness of our D&F method, particularly in scenarios with substantial invisibility, where traditional approaches struggle to maintain reconstruction quality.
MH-pFLGB: Model Heterogeneous personalized Federated Learning via Global Bypass for Medical Image Analysis
Jun 29, 2024Abstract:In the evolving application of medical artificial intelligence, federated learning is notable for its ability to protect training data privacy. Federated learning facilitates collaborative model development without the need to share local data from healthcare institutions. Yet, the statistical and system heterogeneity among these institutions poses substantial challenges, which affects the effectiveness of federated learning and hampers the exchange of information between clients. To address these issues, we introduce a novel approach, MH-pFLGB, which employs a global bypass strategy to mitigate the reliance on public datasets and navigate the complexities of non-IID data distributions. Our method enhances traditional federated learning by integrating a global bypass model, which would share the information among the clients, but also serves as part of the network to enhance the performance on each client. Additionally, MH-pFLGB provides a feature fusion module to better combine the local and global features. We validate \model{}'s effectiveness and adaptability through extensive testing on different medical tasks, demonstrating superior performance compared to existing state-of-the-art methods.
pFLFE: Cross-silo Personalized Federated Learning via Feature Enhancement on Medical Image Segmentation
Jun 29, 2024



Abstract:In medical image segmentation, personalized cross-silo federated learning (FL) is becoming popular for utilizing varied data across healthcare settings to overcome data scarcity and privacy concerns. However, existing methods often suffer from client drift, leading to inconsistent performance and delayed training. We propose a new framework, Personalized Federated Learning via Feature Enhancement (pFLFE), designed to mitigate these challenges. pFLFE consists of two main stages: feature enhancement and supervised learning. The first stage improves differentiation between foreground and background features, and the second uses these enhanced features for learning from segmentation masks. We also design an alternative training approach that requires fewer communication rounds without compromising segmentation quality, even with limited communication resources. Through experiments on three medical segmentation tasks, we demonstrate that pFLFE outperforms the state-of-the-art methods.
MH-pFLID: Model Heterogeneous personalized Federated Learning via Injection and Distillation for Medical Data Analysis
May 10, 2024



Abstract:Federated learning is widely used in medical applications for training global models without needing local data access. However, varying computational capabilities and network architectures (system heterogeneity), across clients pose significant challenges in effectively aggregating information from non-independently and identically distributed (non-IID) data. Current federated learning methods using knowledge distillation require public datasets, raising privacy and data collection issues. Additionally, these datasets require additional local computing and storage resources, which is a burden for medical institutions with limited hardware conditions. In this paper, we introduce a novel federated learning paradigm, named Model Heterogeneous personalized Federated Learning via Injection and Distillation (MH-pFLID). Our framework leverages a lightweight messenger model that carries concentrated information to collect the information from each client. We also develop a set of receiver and transmitter modules to receive and send information from the messenger model, so that the information could be injected and distilled with efficiency.
TRLS: A Time Series Representation Learning Framework via Spectrogram for Medical Signal Processing
Jan 06, 2024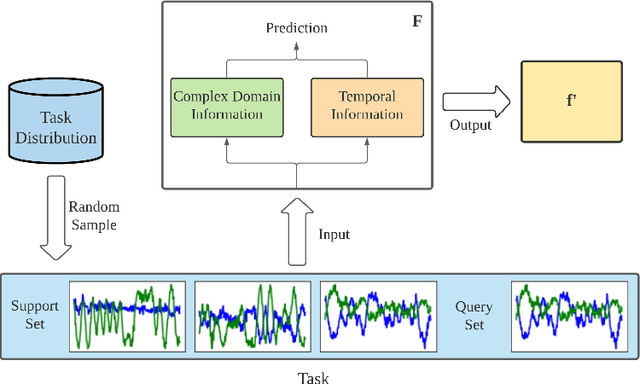
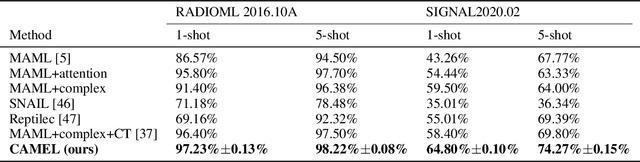

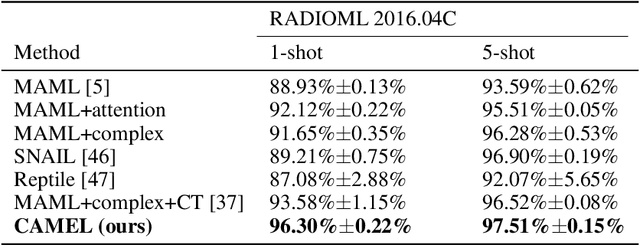
Abstract:Representation learning frameworks in unlabeled time series have been proposed for medical signal processing. Despite the numerous excellent progresses have been made in previous works, we observe the representation extracted for the time series still does not generalize well. In this paper, we present a Time series (medical signal) Representation Learning framework via Spectrogram (TRLS) to get more informative representations. We transform the input time-domain medical signals into spectrograms and design a time-frequency encoder named Time Frequency RNN (TFRNN) to capture more robust multi-scale representations from the augmented spectrograms. Our TRLS takes spectrogram as input with two types of different data augmentations and maximizes the similarity between positive ones, which effectively circumvents the problem of designing negative samples. Our evaluation of four real-world medical signal datasets focusing on medical signal classification shows that TRLS is superior to the existing frameworks.
SHISRCNet: Super-resolution And Classification Network For Low-resolution Breast Cancer Histopathology Image
Jun 25, 2023



Abstract:The rapid identification and accurate diagnosis of breast cancer, known as the killer of women, have become greatly significant for those patients. Numerous breast cancer histopathological image classification methods have been proposed. But they still suffer from two problems. (1) These methods can only hand high-resolution (HR) images. However, the low-resolution (LR) images are often collected by the digital slide scanner with limited hardware conditions. Compared with HR images, LR images often lose some key features like texture, which deeply affects the accuracy of diagnosis. (2) The existing methods have fixed receptive fields, so they can not extract and fuse multi-scale features well for images with different magnification factors. To fill these gaps, we present a \textbf{S}ingle \textbf{H}istopathological \textbf{I}mage \textbf{S}uper-\textbf{R}esolution \textbf{C}lassification network (SHISRCNet), which consists of two modules: Super-Resolution (SR) and Classification (CF) modules. SR module reconstructs LR images into SR ones. CF module extracts and fuses the multi-scale features of SR images for classification. In the training stage, we introduce HR images into the CF module to enhance SHISRCNet's performance. Finally, through the joint training of these two modules, super-resolution and classified of LR images are integrated into our model. The experimental results demonstrate that the effects of our method are close to the SOTA methods with taking HR images as inputs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge