Zhonghua Wang
A Survey of Scientific Large Language Models: From Data Foundations to Agent Frontiers
Aug 28, 2025



Abstract:Scientific Large Language Models (Sci-LLMs) are transforming how knowledge is represented, integrated, and applied in scientific research, yet their progress is shaped by the complex nature of scientific data. This survey presents a comprehensive, data-centric synthesis that reframes the development of Sci-LLMs as a co-evolution between models and their underlying data substrate. We formulate a unified taxonomy of scientific data and a hierarchical model of scientific knowledge, emphasizing the multimodal, cross-scale, and domain-specific challenges that differentiate scientific corpora from general natural language processing datasets. We systematically review recent Sci-LLMs, from general-purpose foundations to specialized models across diverse scientific disciplines, alongside an extensive analysis of over 270 pre-/post-training datasets, showing why Sci-LLMs pose distinct demands -- heterogeneous, multi-scale, uncertainty-laden corpora that require representations preserving domain invariance and enabling cross-modal reasoning. On evaluation, we examine over 190 benchmark datasets and trace a shift from static exams toward process- and discovery-oriented assessments with advanced evaluation protocols. These data-centric analyses highlight persistent issues in scientific data development and discuss emerging solutions involving semi-automated annotation pipelines and expert validation. Finally, we outline a paradigm shift toward closed-loop systems where autonomous agents based on Sci-LLMs actively experiment, validate, and contribute to a living, evolving knowledge base. Collectively, this work provides a roadmap for building trustworthy, continually evolving artificial intelligence (AI) systems that function as a true partner in accelerating scientific discovery.
A General-Purpose Multimodal Foundation Model for Dermatology
Oct 19, 2024



Abstract:Diagnosing and treating skin diseases require advanced visual skills across multiple domains and the ability to synthesize information from various imaging modalities. Current deep learning models, while effective at specific tasks such as diagnosing skin cancer from dermoscopic images, fall short in addressing the complex, multimodal demands of clinical practice. Here, we introduce PanDerm, a multimodal dermatology foundation model pretrained through self-supervised learning on a dataset of over 2 million real-world images of skin diseases, sourced from 11 clinical institutions across 4 imaging modalities. We evaluated PanDerm on 28 diverse datasets covering a range of clinical tasks, including skin cancer screening, phenotype assessment and risk stratification, diagnosis of neoplastic and inflammatory skin diseases, skin lesion segmentation, change monitoring, and metastasis prediction and prognosis. PanDerm achieved state-of-the-art performance across all evaluated tasks, often outperforming existing models even when using only 5-10% of labeled data. PanDerm's clinical utility was demonstrated through reader studies in real-world clinical settings across multiple imaging modalities. It outperformed clinicians by 10.2% in early-stage melanoma detection accuracy and enhanced clinicians' multiclass skin cancer diagnostic accuracy by 11% in a collaborative human-AI setting. Additionally, PanDerm demonstrated robust performance across diverse demographic factors, including different body locations, age groups, genders, and skin tones. The strong results in benchmark evaluations and real-world clinical scenarios suggest that PanDerm could enhance the management of skin diseases and serve as a model for developing multimodal foundation models in other medical specialties, potentially accelerating the integration of AI support in healthcare.
Edge-competing Pathological Liver Vessel Segmentation with Limited Labels
Aug 01, 2021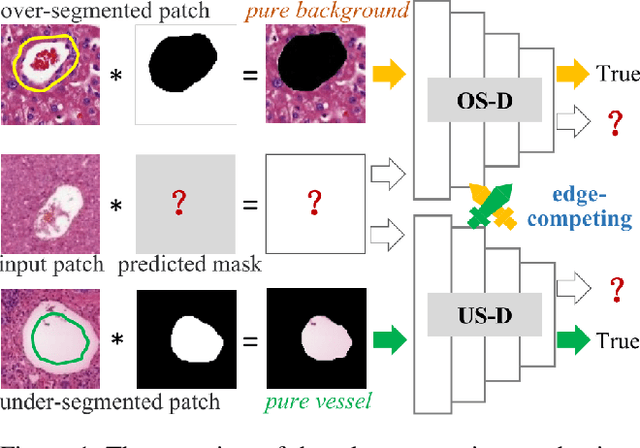
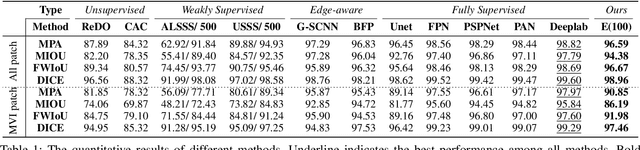
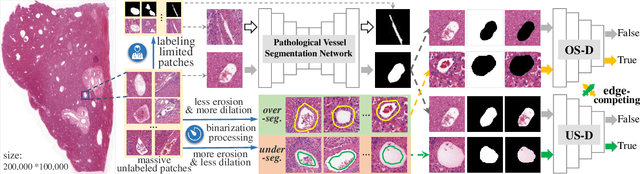

Abstract:The microvascular invasion (MVI) is a major prognostic factor in hepatocellular carcinoma, which is one of the malignant tumors with the highest mortality rate. The diagnosis of MVI needs discovering the vessels that contain hepatocellular carcinoma cells and counting their number in each vessel, which depends heavily on experiences of the doctor, is largely subjective and time-consuming. However, there is no algorithm as yet tailored for the MVI detection from pathological images. This paper collects the first pathological liver image dataset containing 522 whole slide images with labels of vessels, MVI, and hepatocellular carcinoma grades. The first and essential step for the automatic diagnosis of MVI is the accurate segmentation of vessels. The unique characteristics of pathological liver images, such as super-large size, multi-scale vessel, and blurred vessel edges, make the accurate vessel segmentation challenging. Based on the collected dataset, we propose an Edge-competing Vessel Segmentation Network (EVS-Net), which contains a segmentation network and two edge segmentation discriminators. The segmentation network, combined with an edge-aware self-supervision mechanism, is devised to conduct vessel segmentation with limited labeled patches. Meanwhile, two discriminators are introduced to distinguish whether the segmented vessel and background contain residual features in an adversarial manner. In the training stage, two discriminators are devised tocompete for the predicted position of edges. Exhaustive experiments demonstrate that, with only limited labeled patches, EVS-Net achieves a close performance of fully supervised methods, which provides a convenient tool for the pathological liver vessel segmentation. Code is publicly available at https://github.com/zju-vipa/EVS-Net.
BSDA-Net: A Boundary Shape and Distance Aware Joint Learning Framework for Segmenting and Classifying OCTA Images
Jul 13, 2021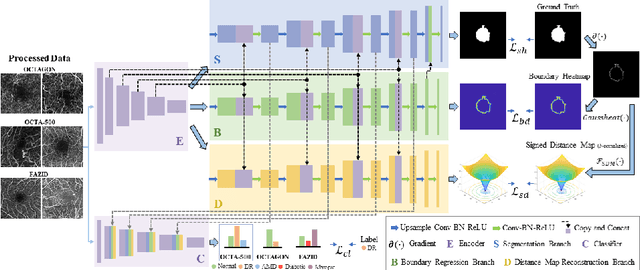
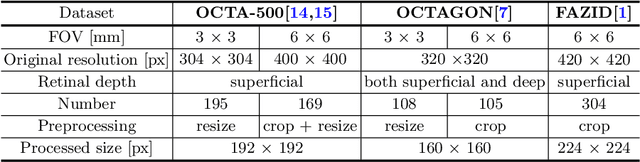
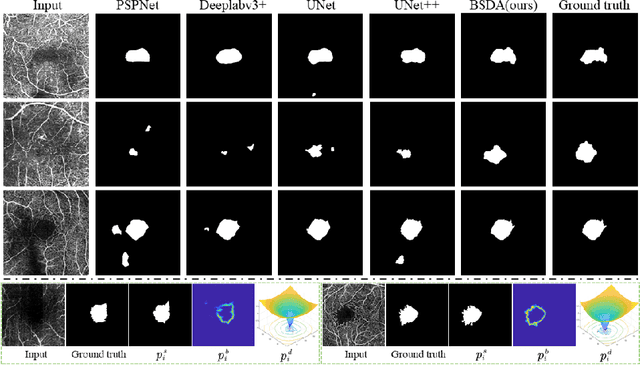
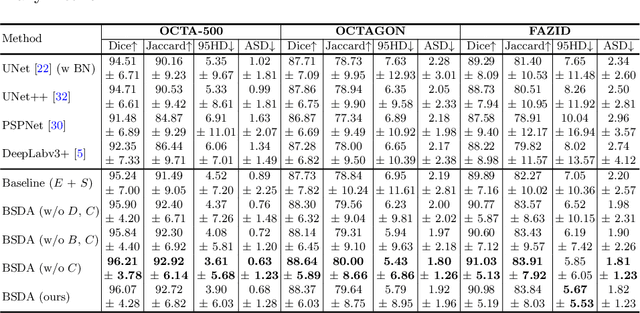
Abstract:Optical coherence tomography angiography (OCTA) is a novel non-invasive imaging technique that allows visualizations of vasculature and foveal avascular zone (FAZ) across retinal layers. Clinical researches suggest that the morphology and contour irregularity of FAZ are important biomarkers of various ocular pathologies. Therefore, precise segmentation of FAZ has great clinical interest. Also, there is no existing research reporting that FAZ features can improve the performance of deep diagnostic classification networks. In this paper, we propose a novel multi-level boundary shape and distance aware joint learning framework, named BSDA-Net, for FAZ segmentation and diagnostic classification from OCTA images. Two auxiliary branches, namely boundary heatmap regression and signed distance map reconstruction branches, are constructed in addition to the segmentation branch to improve the segmentation performance, resulting in more accurate FAZ contours and fewer outliers. Moreover, both low-level and high-level features from the aforementioned three branches, including shape, size, boundary, and signed directional distance map of FAZ, are fused hierarchically with features from the diagnostic classifier. Through extensive experiments, the proposed BSDA-Net is found to yield state-of-the-art segmentation and classification results on the OCTA-500, OCTAGON, and FAZID datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge