Junyan Lyu
Super-Resolution on Rotationally Scanned Photoacoustic Microscopy Images Incorporating Scanning Prior
Dec 12, 2023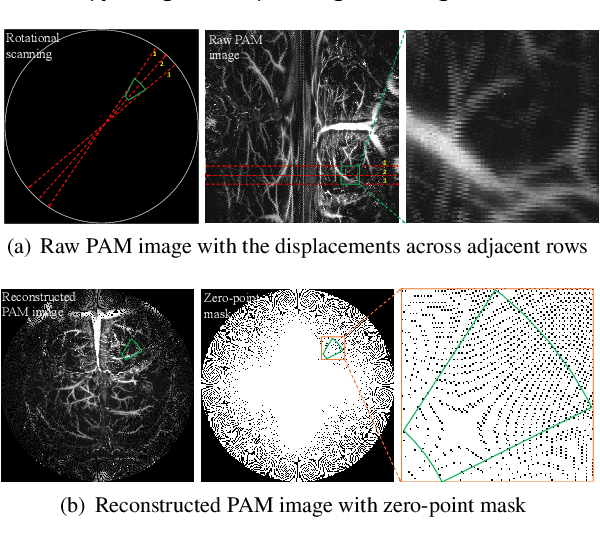
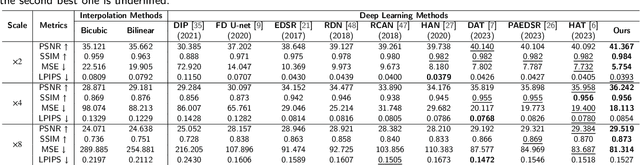
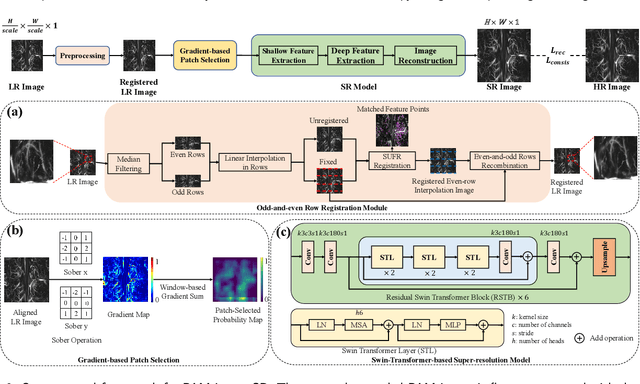
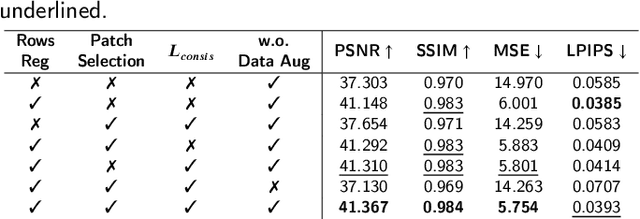
Abstract:Photoacoustic Microscopy (PAM) images integrating the advantages of optical contrast and acoustic resolution have been widely used in brain studies. However, there exists a trade-off between scanning speed and image resolution. Compared with traditional raster scanning, rotational scanning provides good opportunities for fast PAM imaging by optimizing the scanning mechanism. Recently, there is a trend to incorporate deep learning into the scanning process to further increase the scanning speed.Yet, most such attempts are performed for raster scanning while those for rotational scanning are relatively rare. In this study, we propose a novel and well-performing super-resolution framework for rotational scanning-based PAM imaging. To eliminate adjacent rows' displacements due to subject motion or high-frequency scanning distortion,we introduce a registration module across odd and even rows in the preprocessing and incorporate displacement degradation in the training. Besides, gradient-based patch selection is proposed to increase the probability of blood vessel patches being selected for training. A Transformer-based network with a global receptive field is applied for better performance. Experimental results on both synthetic and real datasets demonstrate the effectiveness and generalizability of our proposed framework for rotationally scanned PAM images'super-resolution, both quantitatively and qualitatively. Code is available at https://github.com/11710615/PAMSR.git.
PRIOR: Prototype Representation Joint Learning from Medical Images and Reports
Jul 24, 2023Abstract:Contrastive learning based vision-language joint pre-training has emerged as a successful representation learning strategy. In this paper, we present a prototype representation learning framework incorporating both global and local alignment between medical images and reports. In contrast to standard global multi-modality alignment methods, we employ a local alignment module for fine-grained representation. Furthermore, a cross-modality conditional reconstruction module is designed to interchange information across modalities in the training phase by reconstructing masked images and reports. For reconstructing long reports, a sentence-wise prototype memory bank is constructed, enabling the network to focus on low-level localized visual and high-level clinical linguistic features. Additionally, a non-auto-regressive generation paradigm is proposed for reconstructing non-sequential reports. Experimental results on five downstream tasks, including supervised classification, zero-shot classification, image-to-text retrieval, semantic segmentation, and object detection, show the proposed method outperforms other state-of-the-art methods across multiple datasets and under different dataset size settings. The code is available at https://github.com/QtacierP/PRIOR.
TriFormer: A Multi-modal Transformer Framework For Mild Cognitive Impairment Conversion Prediction
Jul 14, 2023Abstract:The prediction of mild cognitive impairment (MCI) conversion to Alzheimer's disease (AD) is important for early treatment to prevent or slow the progression of AD. To accurately predict the MCI conversion to stable MCI or progressive MCI, we propose Triformer, a novel transformer-based framework with three specialized transformers to incorporate multi-model data. Triformer uses I) an image transformer to extract multi-view image features from medical scans, II) a clinical transformer to embed and correlate multi-modal clinical data, and III) a modality fusion transformer that produces an accurate prediction based on fusing the outputs from the image and clinical transformers. Triformer is evaluated on the Alzheimer's Disease Neuroimaging Initiative (ANDI)1 and ADNI2 datasets and outperforms previous state-of-the-art single and multi-modal methods.
AADG: Automatic Augmentation for Domain Generalization on Retinal Image Segmentation
Jul 27, 2022

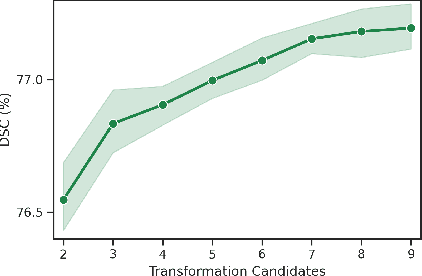
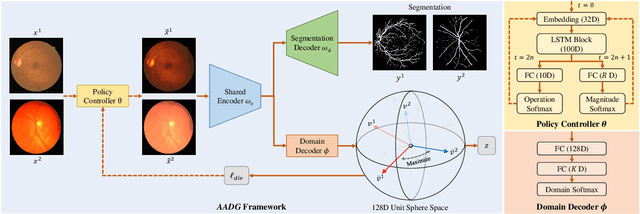
Abstract:Convolutional neural networks have been widely applied to medical image segmentation and have achieved considerable performance. However, the performance may be significantly affected by the domain gap between training data (source domain) and testing data (target domain). To address this issue, we propose a data manipulation based domain generalization method, called Automated Augmentation for Domain Generalization (AADG). Our AADG framework can effectively sample data augmentation policies that generate novel domains and diversify the training set from an appropriate search space. Specifically, we introduce a novel proxy task maximizing the diversity among multiple augmented novel domains as measured by the Sinkhorn distance in a unit sphere space, making automated augmentation tractable. Adversarial training and deep reinforcement learning are employed to efficiently search the objectives. Quantitative and qualitative experiments on 11 publicly-accessible fundus image datasets (four for retinal vessel segmentation, four for optic disc and cup (OD/OC) segmentation and three for retinal lesion segmentation) are comprehensively performed. Two OCTA datasets for retinal vasculature segmentation are further involved to validate cross-modality generalization. Our proposed AADG exhibits state-of-the-art generalization performance and outperforms existing approaches by considerable margins on retinal vessel, OD/OC and lesion segmentation tasks. The learned policies are empirically validated to be model-agnostic and can transfer well to other models. The source code is available at https://github.com/CRazorback/AADG.
Identifying the key components in ResNet-50 for diabetic retinopathy grading from fundus images: a systematic investigation
Oct 27, 2021



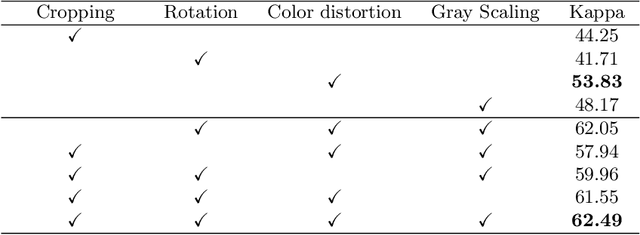
Abstract:Although deep learning based diabetic retinopathy (DR) classification methods typically benefit from well-designed architectures of convolutional neural networks, the training setting also has a non-negligible impact on the prediction performance. The training setting includes various interdependent components, such as objective function, data sampling strategy and data augmentation approach. To identify the key components in a standard deep learning framework (ResNet-50) for DR grading, we systematically analyze the impact of several major components. Extensive experiments are conducted on a publicly-available dataset EyePACS. We demonstrate that (1) the ResNet-50 framework for DR grading is sensitive to input resolution, objective function, and composition of data augmentation, (2) using mean square error as the loss function can effectively improve the performance with respect to a task-specific evaluation metric, namely the quadratically-weighted Kappa, (3) utilizing eye pairs boosts the performance of DR grading and (4) using data resampling to address the problem of imbalanced data distribution in EyePACS hurts the performance. Based on these observations and an optimal combination of the investigated components, our framework, without any specialized network design, achieves the state-of-the-art result (0.8631 for Kappa) on the EyePACS test set (a total of 42670 fundus images) with only image-level labels. Our codes and pre-trained model are available at https://github.com/YijinHuang/pytorch-classification
LDDMM-Face: Large Deformation Diffeomorphic Metric Learning for Flexible and Consistent Face Alignment
Aug 02, 2021



Abstract:We innovatively propose a flexible and consistent face alignment framework, LDDMM-Face, the key contribution of which is a deformation layer that naturally embeds facial geometry in a diffeomorphic way. Instead of predicting facial landmarks via heatmap or coordinate regression, we formulate this task in a diffeomorphic registration manner and predict momenta that uniquely parameterize the deformation between initial boundary and true boundary, and then perform large deformation diffeomorphic metric mapping (LDDMM) simultaneously for curve and landmark to localize the facial landmarks. Due to the embedding of LDDMM into a deep network, LDDMM-Face can consistently annotate facial landmarks without ambiguity and flexibly handle various annotation schemes, and can even predict dense annotations from sparse ones. Our method can be easily integrated into various face alignment networks. We extensively evaluate LDDMM-Face on four benchmark datasets: 300W, WFLW, HELEN and COFW-68. LDDMM-Face is comparable or superior to state-of-the-art methods for traditional within-dataset and same-annotation settings, but truly distinguishes itself with outstanding performance when dealing with weakly-supervised learning (partial-to-full), challenging cases (e.g., occluded faces), and different training and prediction datasets. In addition, LDDMM-Face shows promising results on the most challenging task of predicting across datasets with different annotation schemes.
Lesion-based Contrastive Learning for Diabetic Retinopathy Grading from Fundus Images
Jul 17, 2021
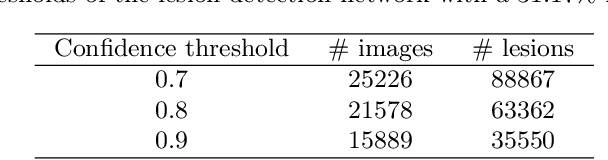
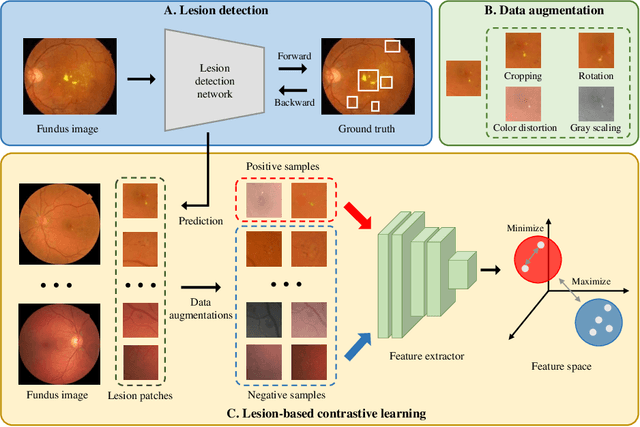
Abstract:Manually annotating medical images is extremely expensive, especially for large-scale datasets. Self-supervised contrastive learning has been explored to learn feature representations from unlabeled images. However, unlike natural images, the application of contrastive learning to medical images is relatively limited. In this work, we propose a self-supervised framework, namely lesion-based contrastive learning for automated diabetic retinopathy (DR) grading. Instead of taking entire images as the input in the common contrastive learning scheme, lesion patches are employed to encourage the feature extractor to learn representations that are highly discriminative for DR grading. We also investigate different data augmentation operations in defining our contrastive prediction task. Extensive experiments are conducted on the publicly-accessible dataset EyePACS, demonstrating that our proposed framework performs outstandingly on DR grading in terms of both linear evaluation and transfer capacity evaluation.
BSDA-Net: A Boundary Shape and Distance Aware Joint Learning Framework for Segmenting and Classifying OCTA Images
Jul 13, 2021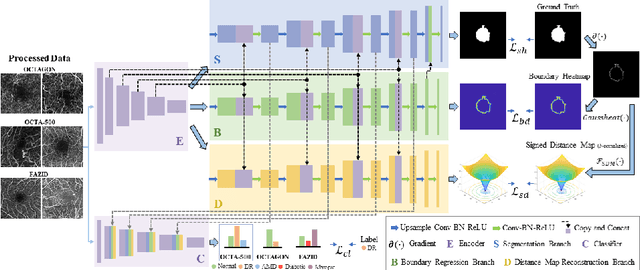
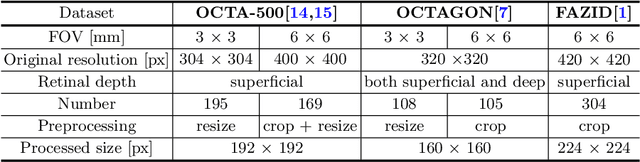
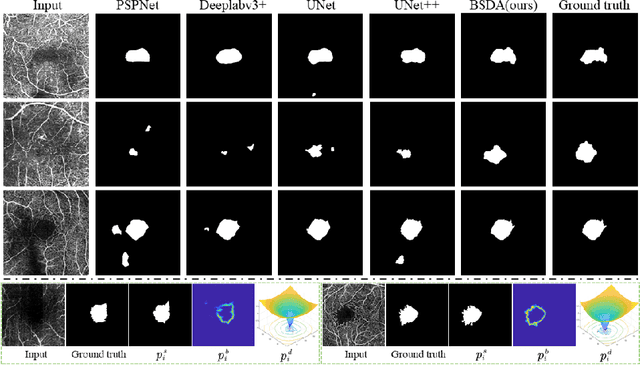
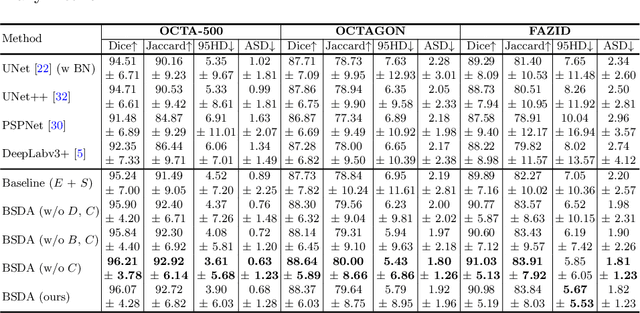
Abstract:Optical coherence tomography angiography (OCTA) is a novel non-invasive imaging technique that allows visualizations of vasculature and foveal avascular zone (FAZ) across retinal layers. Clinical researches suggest that the morphology and contour irregularity of FAZ are important biomarkers of various ocular pathologies. Therefore, precise segmentation of FAZ has great clinical interest. Also, there is no existing research reporting that FAZ features can improve the performance of deep diagnostic classification networks. In this paper, we propose a novel multi-level boundary shape and distance aware joint learning framework, named BSDA-Net, for FAZ segmentation and diagnostic classification from OCTA images. Two auxiliary branches, namely boundary heatmap regression and signed distance map reconstruction branches, are constructed in addition to the segmentation branch to improve the segmentation performance, resulting in more accurate FAZ contours and fewer outliers. Moreover, both low-level and high-level features from the aforementioned three branches, including shape, size, boundary, and signed directional distance map of FAZ, are fused hierarchically with features from the diagnostic classifier. Through extensive experiments, the proposed BSDA-Net is found to yield state-of-the-art segmentation and classification results on the OCTA-500, OCTAGON, and FAZID datasets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge