Litao Yang
A General-Purpose Multimodal Foundation Model for Dermatology
Oct 19, 2024



Abstract:Diagnosing and treating skin diseases require advanced visual skills across multiple domains and the ability to synthesize information from various imaging modalities. Current deep learning models, while effective at specific tasks such as diagnosing skin cancer from dermoscopic images, fall short in addressing the complex, multimodal demands of clinical practice. Here, we introduce PanDerm, a multimodal dermatology foundation model pretrained through self-supervised learning on a dataset of over 2 million real-world images of skin diseases, sourced from 11 clinical institutions across 4 imaging modalities. We evaluated PanDerm on 28 diverse datasets covering a range of clinical tasks, including skin cancer screening, phenotype assessment and risk stratification, diagnosis of neoplastic and inflammatory skin diseases, skin lesion segmentation, change monitoring, and metastasis prediction and prognosis. PanDerm achieved state-of-the-art performance across all evaluated tasks, often outperforming existing models even when using only 5-10% of labeled data. PanDerm's clinical utility was demonstrated through reader studies in real-world clinical settings across multiple imaging modalities. It outperformed clinicians by 10.2% in early-stage melanoma detection accuracy and enhanced clinicians' multiclass skin cancer diagnostic accuracy by 11% in a collaborative human-AI setting. Additionally, PanDerm demonstrated robust performance across diverse demographic factors, including different body locations, age groups, genders, and skin tones. The strong results in benchmark evaluations and real-world clinical scenarios suggest that PanDerm could enhance the management of skin diseases and serve as a model for developing multimodal foundation models in other medical specialties, potentially accelerating the integration of AI support in healthcare.
TPMIL: Trainable Prototype Enhanced Multiple Instance Learning for Whole Slide Image Classification
May 01, 2023Abstract:Digital pathology based on whole slide images (WSIs) plays a key role in cancer diagnosis and clinical practice. Due to the high resolution of the WSI and the unavailability of patch-level annotations, WSI classification is usually formulated as a weakly supervised problem, which relies on multiple instance learning (MIL) based on patches of a WSI. In this paper, we aim to learn an optimal patch-level feature space by integrating prototype learning with MIL. To this end, we develop a Trainable Prototype enhanced deep MIL (TPMIL) framework for weakly supervised WSI classification. In contrast to the conventional methods which rely on a certain number of selected patches for feature space refinement, we softly cluster all the instances by allocating them to their corresponding prototypes. Additionally, our method is able to reveal the correlations between different tumor subtypes through distances between corresponding trained prototypes. More importantly, TPMIL also enables to provide a more accurate interpretability based on the distance of the instances from the trained prototypes which serves as an alternative to the conventional attention score-based interpretability. We test our method on two WSI datasets and it achieves a new SOTA. GitHub repository: https://github.com/LitaoYang-Jet/TPMIL
Leukocyte Classification using Multimodal Architecture Enhanced by Knowledge Distillation
Aug 17, 2022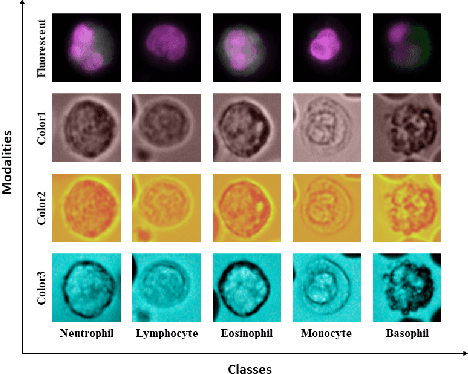
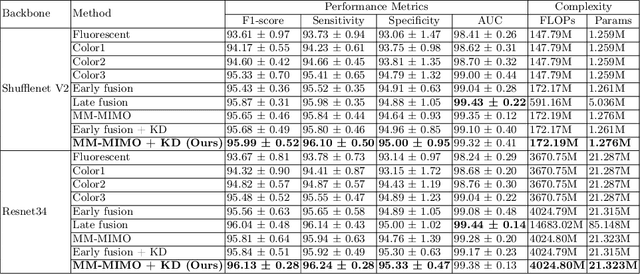
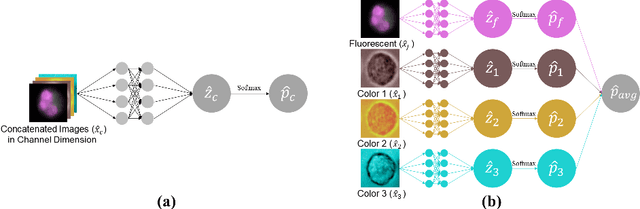
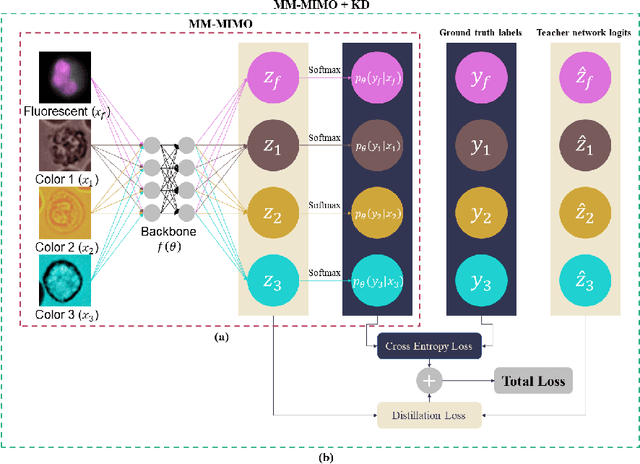
Abstract:Recently, a lot of automated white blood cells (WBC) or leukocyte classification techniques have been developed. However, all of these methods only utilize a single modality microscopic image i.e. either blood smear or fluorescence based, thus missing the potential of a better learning from multimodal images. In this work, we develop an efficient multimodal architecture based on a first of its kind multimodal WBC dataset for the task of WBC classification. Specifically, our proposed idea is developed in two steps - 1) First, we learn modality specific independent subnetworks inside a single network only; 2) We further enhance the learning capability of the independent subnetworks by distilling knowledge from high complexity independent teacher networks. With this, our proposed framework can achieve a high performance while maintaining low complexity for a multimodal dataset. Our unique contribution is two-fold - 1) We present a first of its kind multimodal WBC dataset for WBC classification; 2) We develop a high performing multimodal architecture which is also efficient and low in complexity at the same time.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge