Wiro Niessen
for the Heart-Brain Connection Consortium
AngioMoCo: Learning-based Motion Correction in Cerebral Digital Subtraction Angiography
Oct 09, 2023Abstract:Cerebral X-ray digital subtraction angiography (DSA) is the standard imaging technique for visualizing blood flow and guiding endovascular treatments. The quality of DSA is often negatively impacted by body motion during acquisition, leading to decreased diagnostic value. Time-consuming iterative methods address motion correction based on non-rigid registration, and employ sparse key points and non-rigidity penalties to limit vessel distortion. Recent methods alleviate subtraction artifacts by predicting the subtracted frame from the corresponding unsubtracted frame, but do not explicitly compensate for motion-induced misalignment between frames. This hinders the serial evaluation of blood flow, and often causes undesired vasculature and contrast flow alterations, leading to impeded usability in clinical practice. To address these limitations, we present AngioMoCo, a learning-based framework that generates motion-compensated DSA sequences from X-ray angiography. AngioMoCo integrates contrast extraction and motion correction, enabling differentiation between patient motion and intensity changes caused by contrast flow. This strategy improves registration quality while being substantially faster than iterative elastix-based methods. We demonstrate AngioMoCo on a large national multi-center dataset (MR CLEAN Registry) of clinically acquired angiographic images through comprehensive qualitative and quantitative analyses. AngioMoCo produces high-quality motion-compensated DSA, removing motion artifacts while preserving contrast flow. Code is publicly available at https://github.com/RuishengSu/AngioMoCo.
Prior-knowledge-informed deep learning for lacune detection and quantification using multi-site brain MRI
Jun 18, 2023

Abstract:Lacunes of presumed vascular origin, also referred to as lacunar infarcts, are important to assess cerebral small vessel disease and cognitive diseases such as dementia. However, visual rating of lacunes from imaging data is challenging, time-consuming, and rater-dependent, owing to their small size, sparsity, and mimics. Whereas recent developments in automatic algorithms have shown to make the detection of lacunes faster while preserving sensitivity, they also showed a large number of false positives, which makes them impractical for use in clinical practice or large-scale studies. Here, we develop a novel framework that, in addition to lacune detection, outputs a categorical burden score. This score could provide a more practical estimate of lacune presence that simplifies and effectively accelerates the imaging assessment of lacunes. We hypothesize that the combination of detection and the categorical score makes the procedure less sensitive to noisy labels.
Spatio-Temporal U-Net for Cerebral Artery and Vein Segmentation in Digital Subtraction Angiography
Aug 03, 2022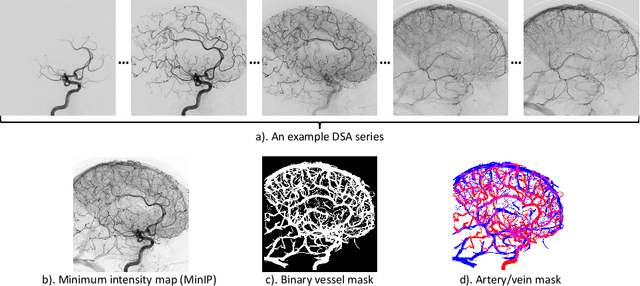
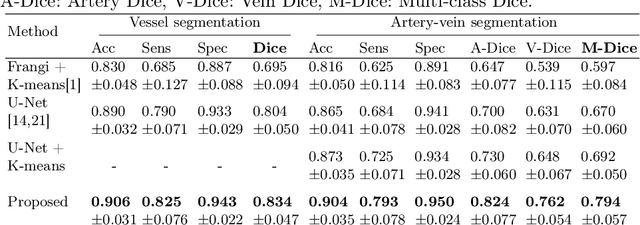
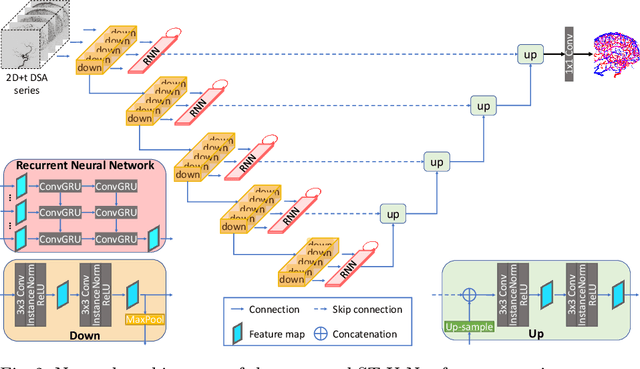
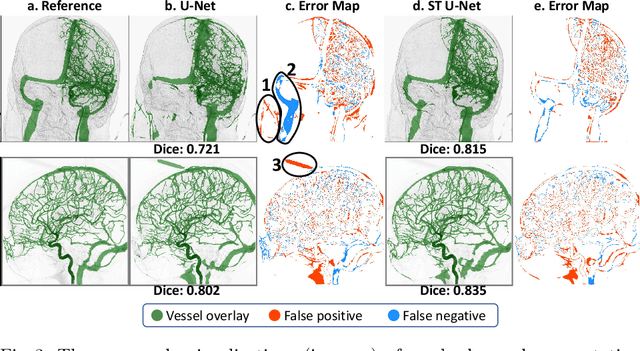
Abstract:X-ray digital subtraction angiography (DSA) is widely used for vessel and/or flow visualization and interventional guidance during endovascular treatment of patients with a stroke or aneurysm. To assist in peri-operative decision making as well as post-operative prognosis, automatic DSA analysis algorithms are being developed to obtain relevant image-based information. Such analyses include detection of vascular disease, evaluation of perfusion based on time intensity curves (TIC), and quantitative biomarker extraction for automated treatment evaluation in endovascular thrombectomy. Methodologically, such vessel-based analysis tasks may be facilitated by automatic and accurate artery-vein segmentation algorithms. The present work describes to the best of our knowledge the first study that addresses automatic artery-vein segmentation in DSA using deep learning. We propose a novel spatio-temporal U-Net (ST U-Net) architecture which integrates convolutional gated recurrent units (ConvGRU) in the contracting branch of U-Net. The network encodes a 2D+t DSA series of variable length and decodes it into a 2D segmentation image. On a multi-center routinely acquired dataset, the proposed method significantly outperformed U-Net (P<0.001) and traditional Frangi-based K-means clustering (P$<$0.001). Particularly in artery-vein segmentation, ST U-Net achieved a Dice coefficient of 0.794, surpassing the existing state-of-the-art methods by a margin of 12\%-20\%. Code will be made publicly available upon acceptance.
Projection-wise Disentangling for Fair and Interpretable Representation Learning: Application to 3D Facial Shape Analysis
Jun 28, 2021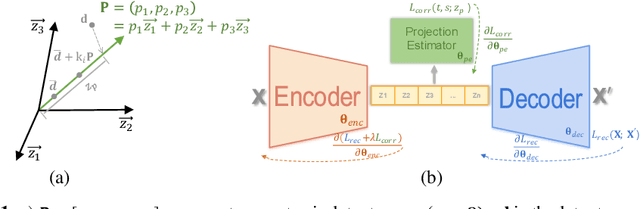
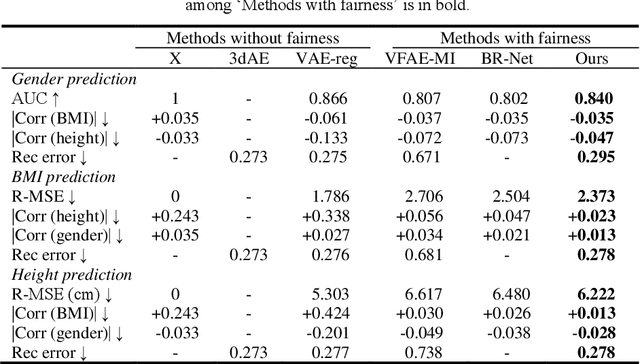
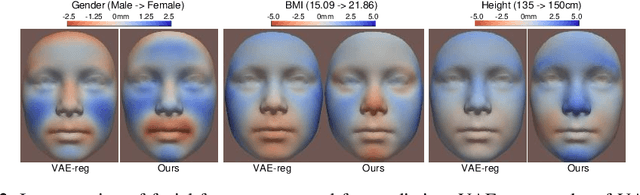
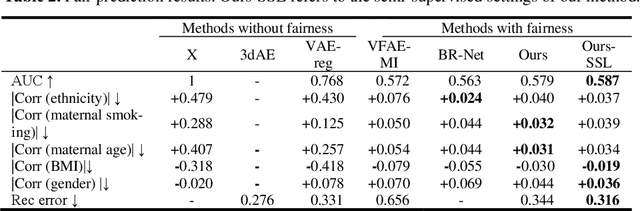
Abstract:Confounding bias is a crucial problem when applying machine learning to practice, especially in clinical practice. We consider the problem of learning representations independent to multiple biases. In literature, this is mostly solved by purging the bias information from learned representations. We however expect this strategy to harm the diversity of information in the representation, and thus limiting its prospective usage (e.g., interpretation). Therefore, we propose to mitigate the bias while keeping almost all information in the latent representations, which enables us to observe and interpret them as well. To achieve this, we project latent features onto a learned vector direction, and enforce the independence between biases and projected features rather than all learned features. To interpret the mapping between projected features and input data, we propose projection-wise disentangling: a sampling and reconstruction along the learned vector direction. The proposed method was evaluated on the analysis of 3D facial shape and patient characteristics (N=5011). Experiments showed that this conceptually simple method achieved state-of-the-art fair prediction performance and interpretability, showing its great potential for clinical applications.
Automated Estimation of the Spinal Curvature via Spine Centerline Extraction with Ensembles of Cascaded Neural Networks
Dec 11, 2019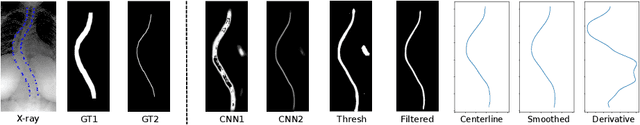

Abstract:Scoliosis is a condition defined by an abnormal spinal curvature. For diagnosis and treatment planning of scoliosis, spinal curvature can be estimated using Cobb angles. We propose an automated method for the estimation of Cobb angles from X-ray scans. First, the centerline of the spine was segmented using a cascade of two convolutional neural networks. After smoothing the centerline, Cobb angles were automatically estimated using the derivative of the centerline. We evaluated the results using the mean absolute error and the average symmetric mean absolute percentage error between the manual assessment by experts and the automated predictions. For optimization, we used 609 X-ray scans from the London Health Sciences Center, and for evaluation, we participated in the international challenge "Accurate Automated Spinal Curvature Estimation, MICCAI 2019" (100 scans). On the challenge's test set, we obtained an average symmetric mean absolute percentage error of 22.96.
Reproducible White Matter Tract Segmentation Using 3D U-Net on a Large-scale DTI Dataset
Aug 26, 2019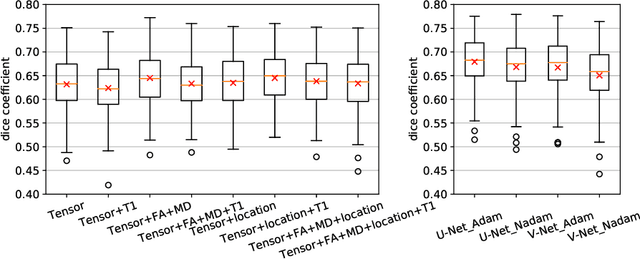
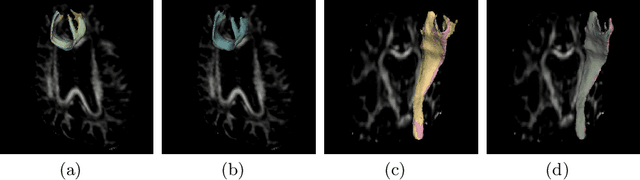
Abstract:Tract-specific diffusion measures, as derived from brain diffusion MRI, have been linked to white matter tract structural integrity and neurodegeneration. As a consequence, there is a large interest in the automatic segmentation of white matter tract in diffusion tensor MRI data. Methods based on the tractography are popular for white matter tract segmentation. However, because of the limited consistency and long processing time, such methods may not be suitable for clinical practice. We therefore developed a novel convolutional neural network based method to directly segment white matter tract trained on a low-resolution dataset of 9149 DTI images. The method is optimized on input, loss function and network architecture selections. We evaluated both segmentation accuracy and reproducibility, and reproducibility of determining tract-specific diffusion measures. The reproducibility of the method is higher than that of the reference standard and the determined diffusion measures are consistent. Therefore, we expect our method to be applicable in clinical practice and in longitudinal analysis of white matter microstructure.
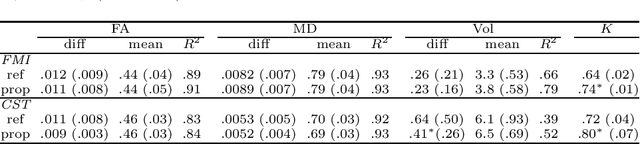
A hybrid deep learning framework for integrated segmentation and registration: evaluation on longitudinal white matter tract changes
Aug 26, 2019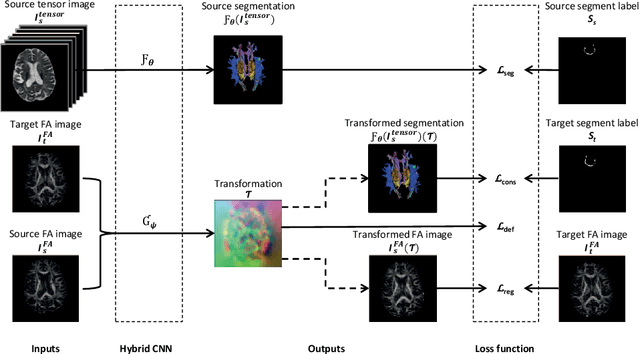
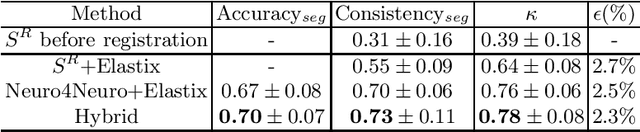
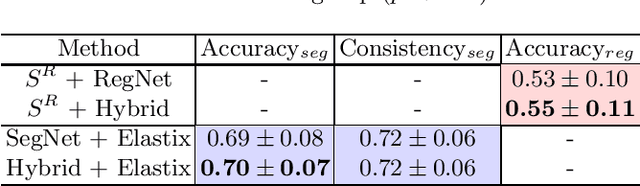
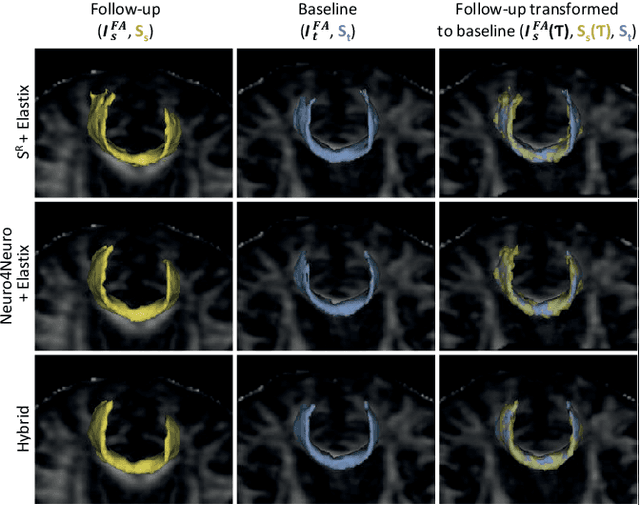
Abstract:To accurately analyze changes of anatomical structures in longitudinal imaging studies, consistent segmentation across multiple time-points is required. Existing solutions often involve independent registration and segmentation components. Registration between time-points is used either as a prior for segmentation in a subsequent time point or to perform segmentation in a common space. In this work, we propose a novel hybrid convolutional neural network (CNN) that integrates segmentation and registration into a single procedure. We hypothesize that the joint optimization leads to increased performance on both tasks. The hybrid CNN is trained by minimizing an integrated loss function composed of four different terms, measuring segmentation accuracy, similarity between registered images, deformation field smoothness, and segmentation consistency. We applied this method to the segmentation of white matter tracts, describing functionally grouped axonal fibers, using N=8045 longitudinal brain MRI data of 3249 individuals. The proposed method was compared with two multistage pipelines using two existing segmentation methods combined with a conventional deformable registration algorithm. In addition, we assessed the added value of the joint optimization for segmentation and registration separately. The hybrid CNN yielded significantly higher accuracy, consistency and reproducibility of segmentation than the multistage pipelines, and was orders of magnitude faster. Therefore, we expect it can serve as a novel tool to support clinical and epidemiological analyses on understanding microstructural brain changes over time.
Automated Image Registration Quality Assessment Utilizing Deep-learning based Ventricle Extraction in Clinical Data
Jul 01, 2019

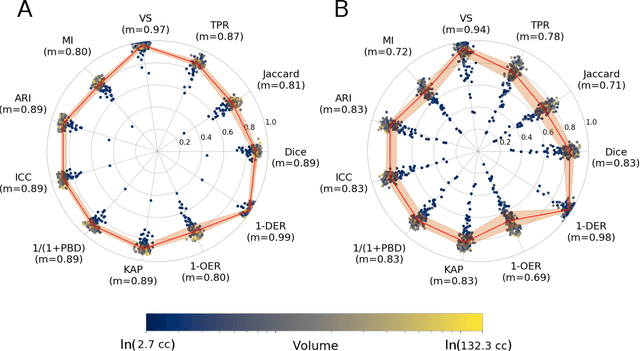
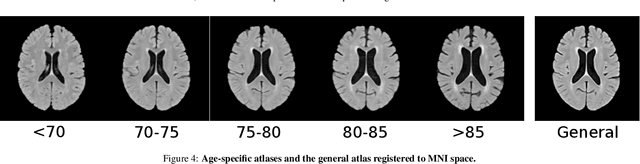
Abstract:Registration is a core component of many imaging pipelines. In case of clinical scans, with lower resolution and sometimes substantial motion artifacts, registration can produce poor results. Visual assessment of registration quality in large clinical datasets is inefficient. In this work, we propose to automatically assess the quality of registration to an atlas in clinical FLAIR MRI scans of the brain. The method consists of automatically segmenting the ventricles of a given scan using a neural network, and comparing the segmentation to the atlas' ventricles propagated to image space. We used the proposed method to improve clinical image registration to a general atlas by computing multiple registrations and then selecting the registration that yielded the highest ventricle overlap. Methods were evaluated in a single-site dataset of more than 1000 scans, as well as a multi-center dataset comprising 142 clinical scans from 12 sites. The automated ventricle segmentation reached a Dice coefficient with manual annotations of 0.89 in the single-site dataset, and 0.83 in the multi-center dataset. Registration via age-specific atlases could improve ventricle overlap compared to a direct registration to the general atlas (Dice similarity coefficient increase up to 0.15). Experiments also showed that selecting scans with the registration quality assessment method could improve the quality of average maps of white matter hyperintensity burden, instead of using all scans for the computation of the white matter hyperintensity map. In this work, we demonstrated the utility of an automated tool for assessing image registration quality in clinical scans. This image quality assessment step could ultimately assist in the translation of automated neuroimaging pipelines to the clinic.
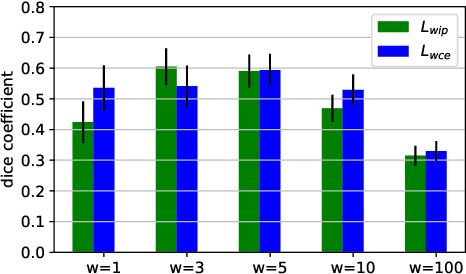
Weakly Supervised Object Detection with 2D and 3D Regression Neural Networks
Jun 14, 2019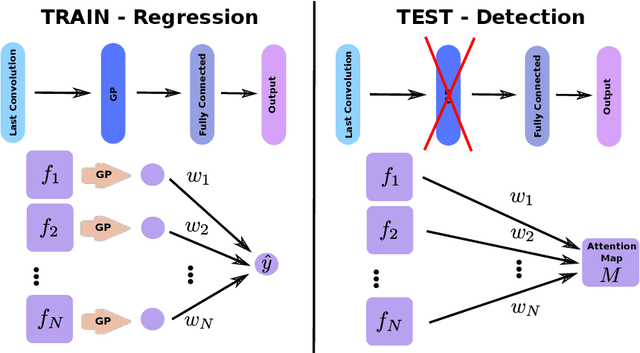
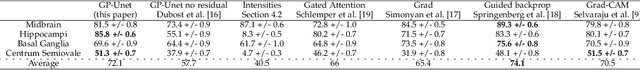
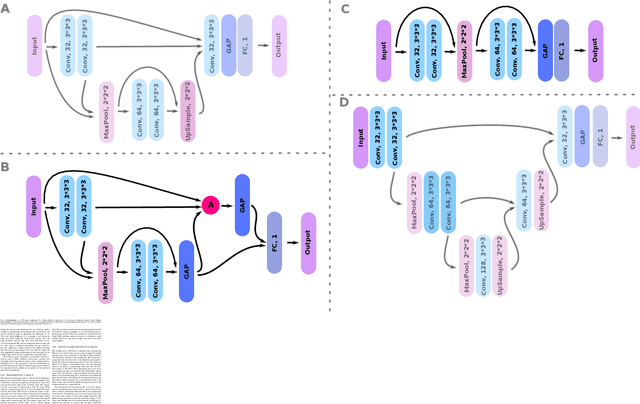
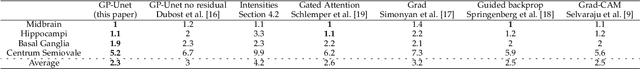
Abstract:Weakly supervised detection methods can infer the location of target objects in an image without requiring location or appearance information during training. We propose a weakly supervised deep learning method for the detection of objects that appear at multiple locations in an image. The method computes attention maps using the last feature maps of an encoder-decoder network optimized only with global labels: the number of occurrences of the target object in an image. In contrast with previous approaches, attention maps are generated at full input resolution thanks to the decoder part. The proposed approach is compared to multiple state-of-the-art methods in two tasks: the detection of digits in MNIST-based datasets, and the real life application of detection of enlarged perivascular spaces -- a type of brain lesion -- in four brain regions in a dataset of 2202 3D brain MRI scans. In MNIST-based datasets, the proposed method outperforms the other methods. In the brain dataset, several weakly supervised detection methods come close to the human intrarater agreement in each region. The proposed method reaches the lowest number of false positive detections in all brain regions at the operating point, while its average sensitivity is similar to that of the other best methods.
3D Regression Neural Network for the Quantification of Enlarged Perivascular Spaces in Brain MRI
Oct 28, 2018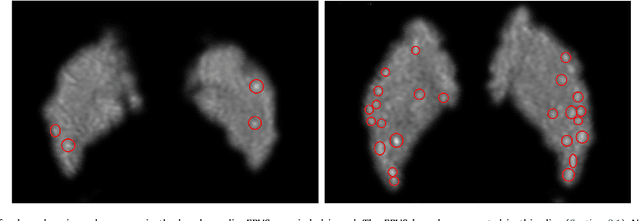
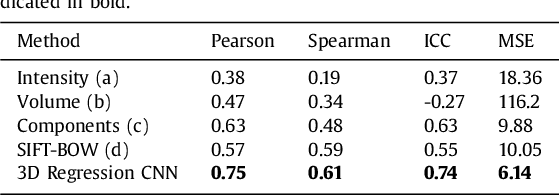
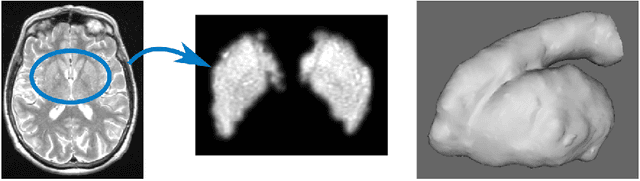
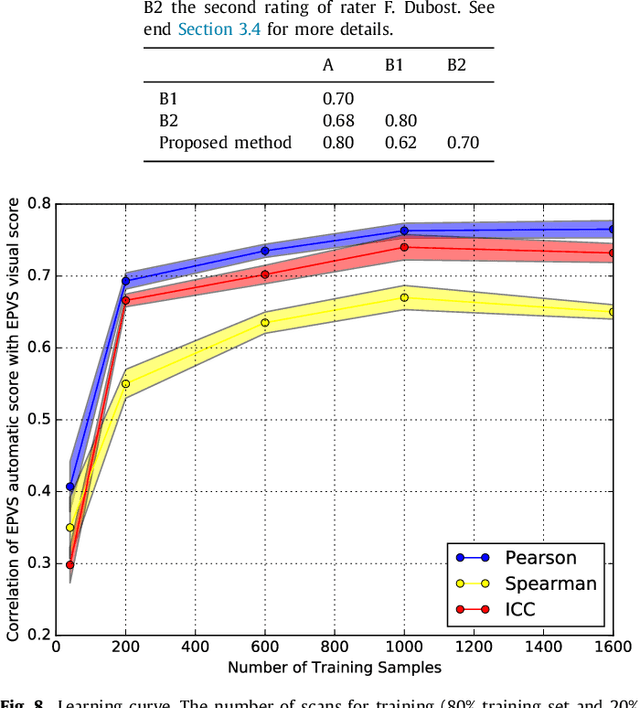
Abstract:Enlarged perivascular spaces (EPVS) in the brain are an emerging imaging marker for cerebral small vessel disease, and have been shown to be related to increased risk of various neurological diseases, including stroke and dementia. Automatic quantification of EPVS would greatly help to advance research into its etiology and its potential as a risk indicator of disease. We propose a convolutional network regression method to quantify the extent of EPVS in the basal ganglia from 3D brain MRI. We first segment the basal ganglia and subsequently apply a 3D convolutional regression network designed for small object detection within this region of interest. The network takes an image as input, and outputs a quantification score of EPVS. The network has significantly more convolution operations than pooling ones and no final activation, allowing it to span the space of real numbers. We validated our approach using a dataset of 2000 brain MRI scans scored visually. Experiments with varying sizes of training and test sets showed that a good performance can be achieved with a training set of only 200 scans. With a training set of 1000 scans, the intraclass correlation coefficient (ICC) between our scoring method and the expert's visual score was 0.74. Our method outperforms by a large margin - more than 0.10 - four more conventional automated approaches based on intensities, scale-invariant feature transform, and random forest. We show that the network learns the structures of interest and investigate the influence of hyper-parameters on the performance. We also evaluate the reproducibility of our network using a set of 60 subjects scanned twice (scan-rescan reproducibility). On this set our network achieves an ICC of 0.93, while the intrarater agreement reaches 0.80. Furthermore, the automatic EPVS scoring correlates similarly to age as visual scoring.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge